Enzymes Making Lifes Reactions Possible Terminology Reactants The

Enzymes Making Life’s Reactions Possible
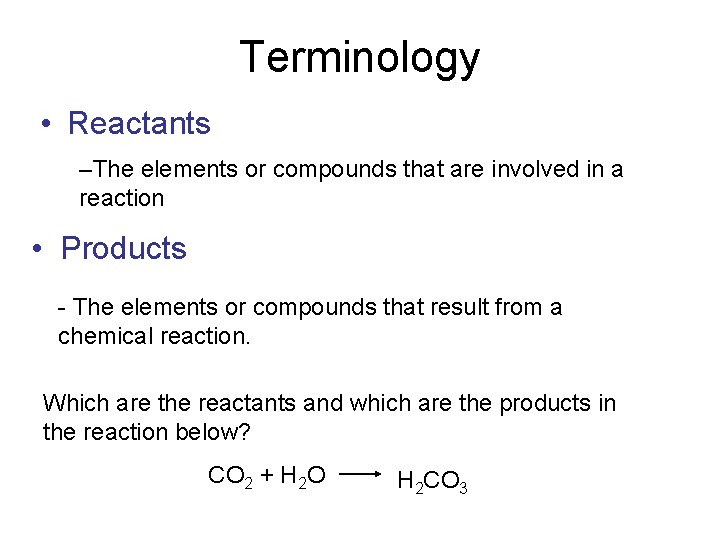
Terminology • Reactants –The elements or compounds that are involved in a reaction • Products - The elements or compounds that result from a chemical reaction. Which are the reactants and which are the products in the reaction below? CO 2 + H 2 O H 2 CO 3

Chemical Reactions • Chemical reactions can either release energy or absorb energy. • Reactions that release energy often occur spontaneously. Hydrogen and oxygen combining to form water is an energy releasing reaction. • Reactions that absorb energy often require an input of energy to occur. Water breaking down into hydrogen and oxygen requires electricity in order to occur.
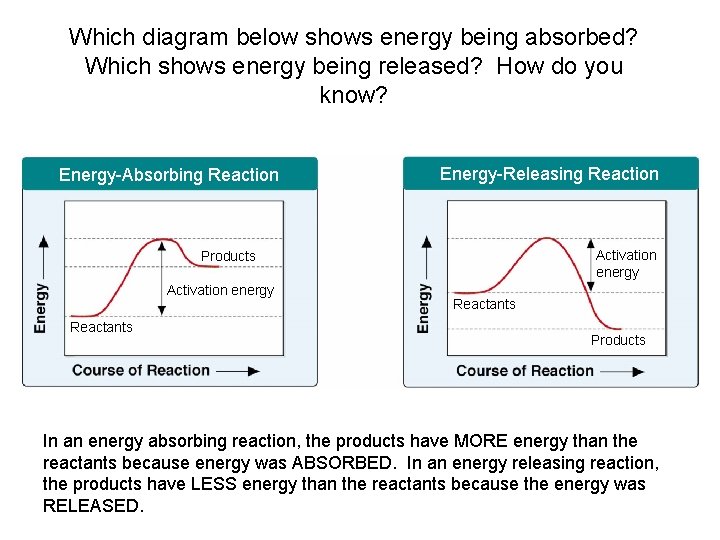
Which diagram below shows energy being absorbed? Which Section 2 -4 shows energy being released? How do you know? Energy-Absorbing Reaction Energy-Releasing Reaction Activation energy Products Activation energy Reactants Products In an energy absorbing reaction, the products have MORE energy than the reactants because energy was ABSORBED. In an energy releasing reaction, the products have LESS energy than the reactants because the energy was Go to. RELEASED. Section:

Activation Energy • Not all reactions occur spontaneously, not even those that release energy. Many reactions need energy in order to begin. This energy is called activation energy.
Catalysts • A catalyst is a substance that speeds up a reaction, but that is not used up in the reaction. • Enzymes are proteins that cells use as biological catalysts. • Cells use enzymes to speed up the chemical reactions that take place in cells.
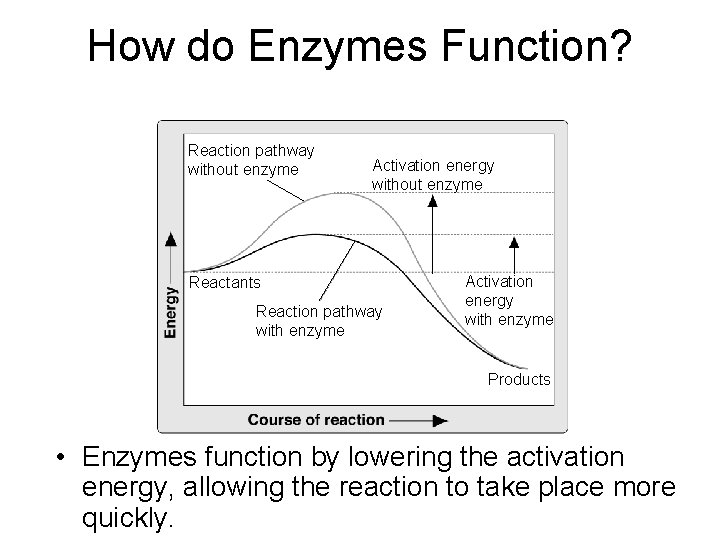
How do Enzymes Function? Reaction pathway without enzyme Activation energy without enzyme Reactants Reaction pathway with enzyme Activation energy with enzyme Products • Enzymes function by lowering the activation energy, allowing the reaction to take place more Go to Section: quickly.
Enzyme Facts • In the reaction, CO 2 + H 2 O H 2 CO 3, the enzyme carbonic anhydrase speeds up the reaction by a factor of 10 million, allowing carbon dioxide to be transferred efficiently to the blood for removal at the lungs. • Enzymes are very specific, generally catalyzing only one chemical reaction. Because of this, part of the enzyme’s name comes from the reaction it catalyzes.

The Enzyme Substrate Complex • Substrate: Another name for the reactant in a reaction involving an enzyme. • Active Site: The specific area on an enzyme where the substrate attaches. This area is so specific that the enzyme is often referred to as a lock, and the substrate as the key.
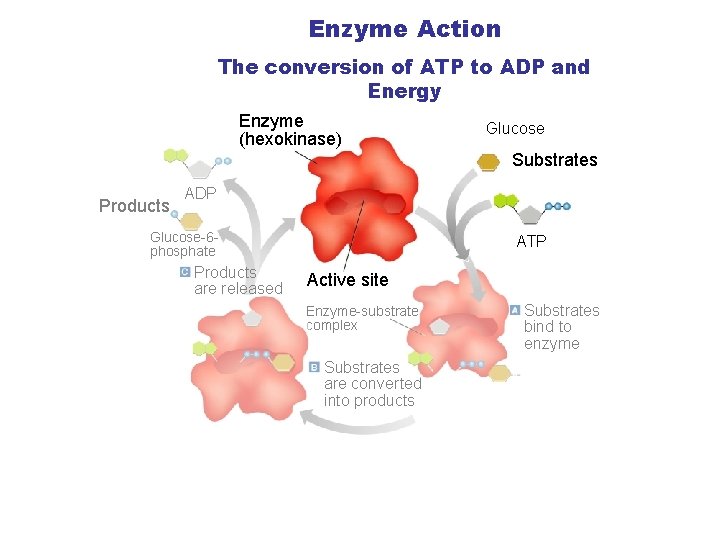
Enzyme Action The conversion of ATP to ADP and Energy Section 2 -4 Enzyme (hexokinase) Glucose Substrates Products ADP Glucose-6 phosphate Products are released ATP Active site Enzyme-substrate complex Substrates are converted into products Go to Section: Substrates bind to enzyme
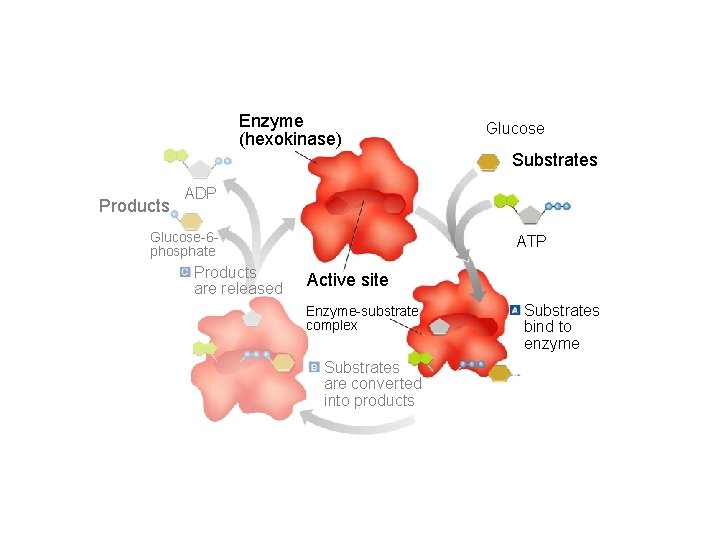
Section 2 -4 Enzyme (hexokinase) Glucose Substrates Products ADP Glucose-6 phosphate Products are released ATP Active site Enzyme-substrate complex Substrates are converted into products Go to Section: Substrates bind to enzyme
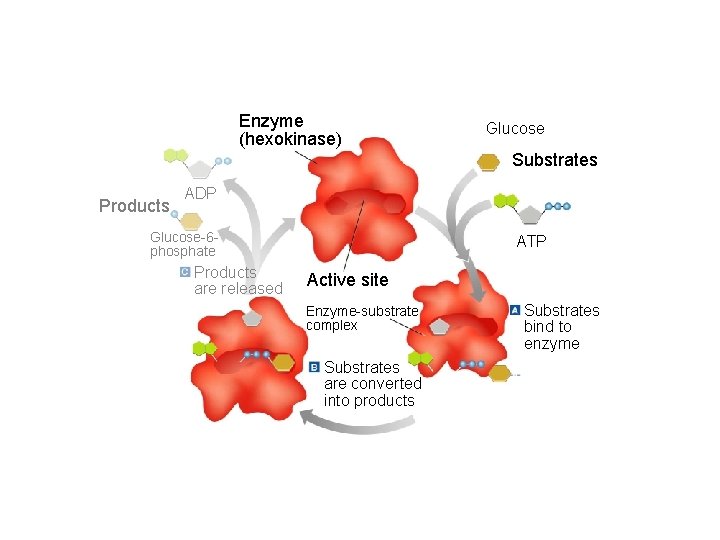
Section 2 -4 Enzyme (hexokinase) Glucose Substrates Products ADP Glucose-6 phosphate Products are released ATP Active site Enzyme-substrate complex Substrates are converted into products Go to Section: Substrates bind to enzyme
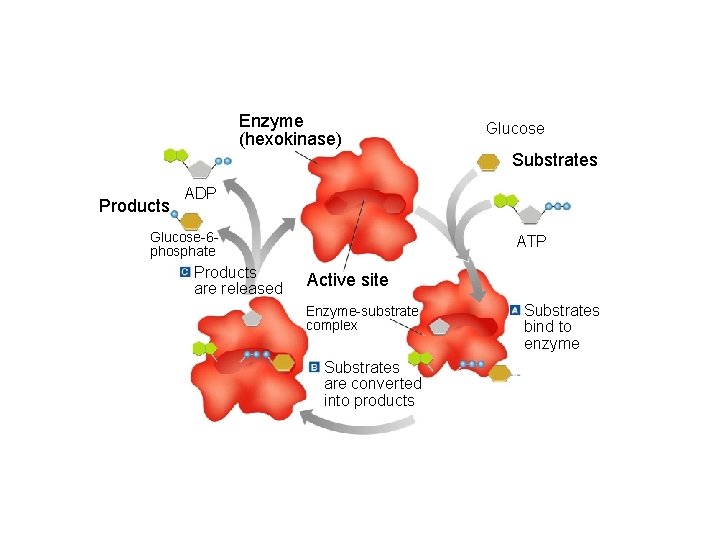
Figure 2 -21 Enzyme Action Section 2 -4 Enzyme (hexokinase) Glucose Substrates Products ADP Glucose-6 phosphate Products are released ATP Active site Enzyme-substrate complex Substrates are converted into products Go to Section: Substrates bind to enzyme
What Affects Enzyme Activity? • p. H • Temperature: Most enzymes work best at 37 C.
The Role of Enzymes • Enzymes regulate chemical pathways, resulting in materials that the cells need. • Enzymes play roles in releasing and storing energy. • Enzymes help to transfer information.
- Slides: 15