Enzymes Helper Protein molecules Chemical reactions of life


- Slides: 19

Enzymes: “Helper” Protein molecules

Chemical reactions of life • Processes of life require: – building molecules • Synthesis (dehydration synthesis) ; water is released – breaking down molecules • Digestion (hydrolysis): water is required

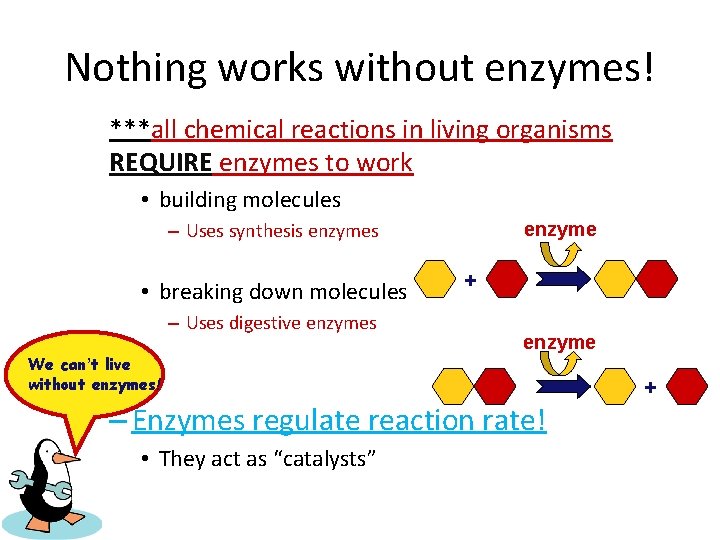
Nothing works without enzymes! ***all chemical reactions in living organisms REQUIRE enzymes to work • building molecules enzyme – Uses synthesis enzymes • breaking down molecules – Uses digestive enzymes We can’t live without enzymes! + enzyme – Enzymes regulate reaction rate! • They act as “catalysts” +

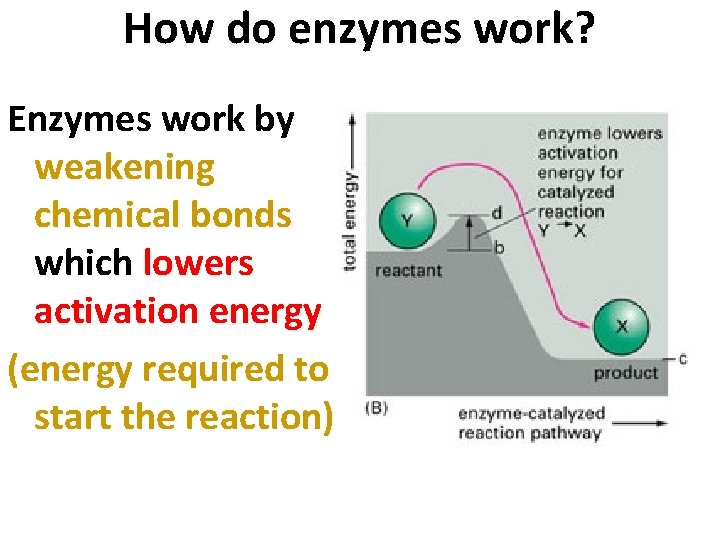
How do enzymes work? Enzymes work by weakening chemical bonds which lowers activation energy (energy required to start the reaction) 4

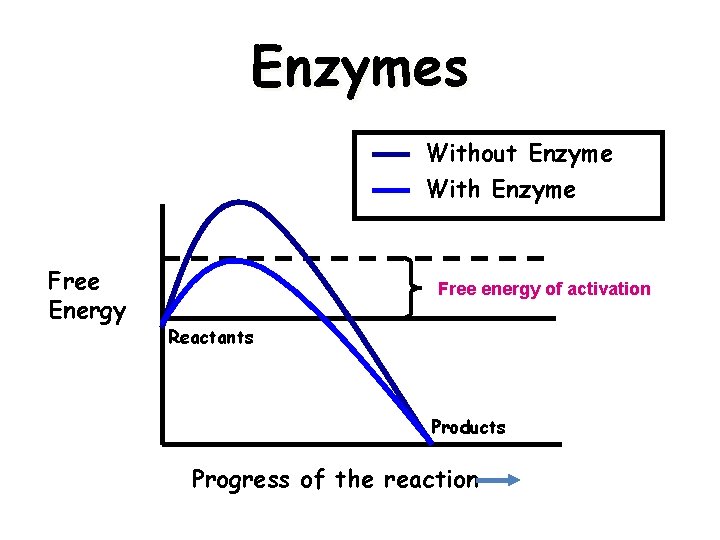
Enzymes Without Enzyme With Enzyme Free Energy Free energy of activation Reactants Products Progress of the reaction 5

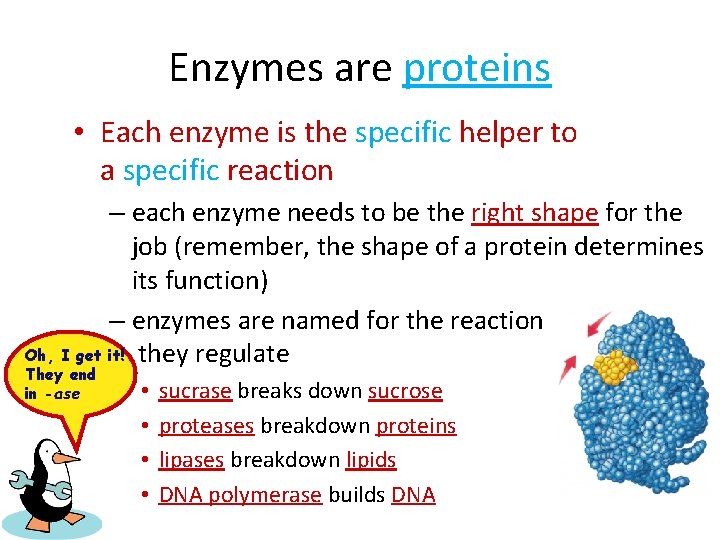
Enzymes are proteins • Each enzyme is the specific helper to a specific reaction – each enzyme needs to be the right shape for the job (remember, the shape of a protein determines its function) – enzymes are named for the reaction Oh, I get it! they regulate They end in -ase • • sucrase breaks down sucrose proteases breakdown proteins lipases breakdown lipids DNA polymerase builds DNA

Enzyme Characteristics: • Enzymes are not changed by the reaction – used only temporarily – released and re-used again for the same reaction with other molecules of the same type – very little enzyme needed to facilitate many reactions

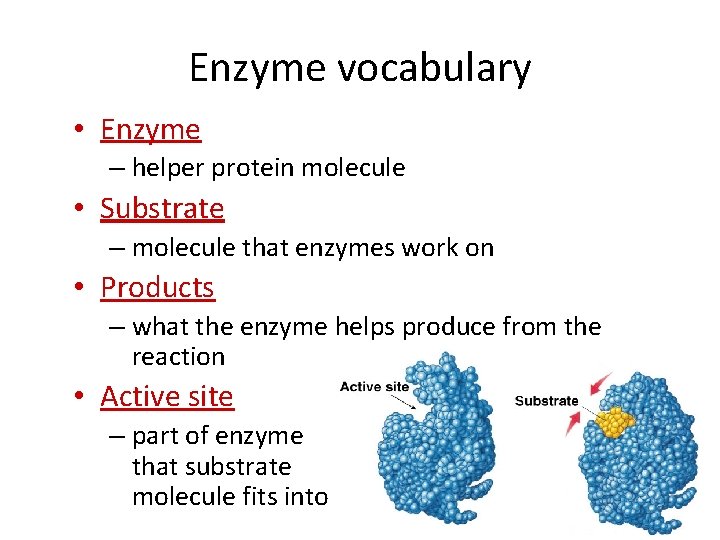
Enzyme vocabulary • Enzyme – helper protein molecule • Substrate – molecule that enzymes work on • Products – what the enzyme helps produce from the reaction • Active site – part of enzyme that substrate molecule fits into

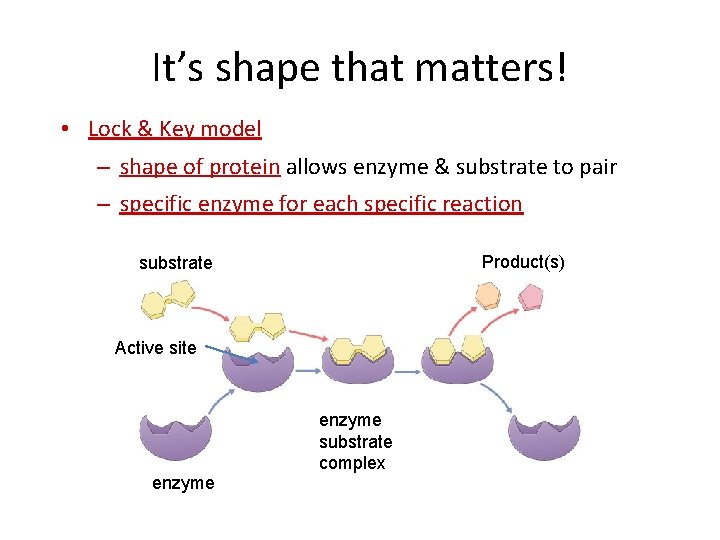
It’s shape that matters! • Lock & Key model – shape of protein allows enzyme & substrate to pair – specific enzyme for each specific reaction Product(s) substrate Active site enzyme substrate complex enzyme

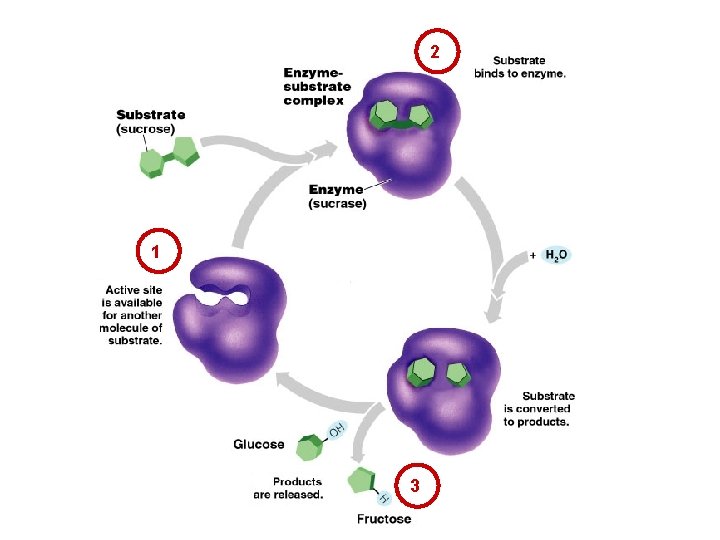
2 1 3

What affects enzyme action • Correct protein structure – Must have the correct order of amino acids – why? enzyme has to be right shape • Temperature – why? enzyme has to be right shape • p. H (acids & bases) – why? enzyme has to be right shape

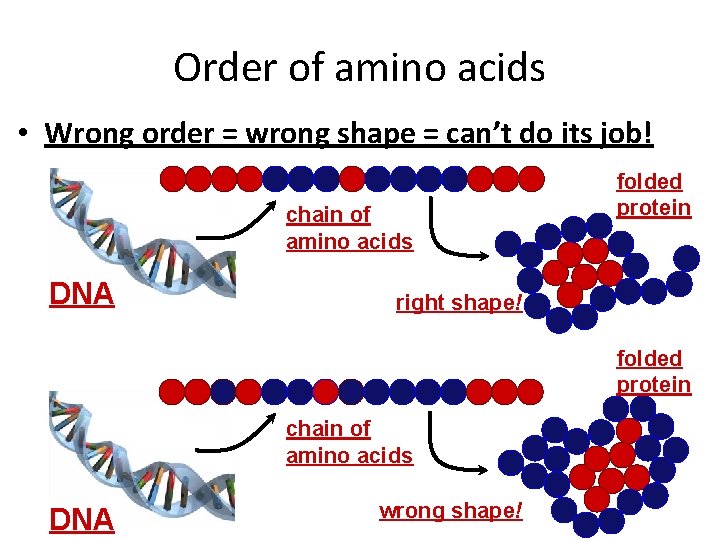
Order of amino acids • Wrong order = wrong shape = can’t do its job! chain of amino acids DNA folded protein right shape! folded protein chain of amino acids DNA wrong shape!

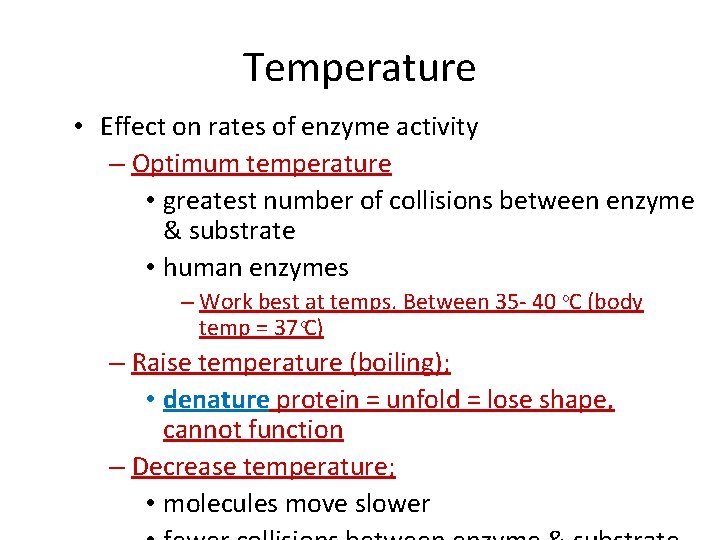
Temperature • Effect on rates of enzyme activity – Optimum temperature • greatest number of collisions between enzyme & substrate • human enzymes – Work best at temps. Between 35 - 40 ᵒC (body temp = 37 C) – Raise temperature (boiling); • denature protein = unfold = lose shape, cannot function – Decrease temperature; • molecules move slower

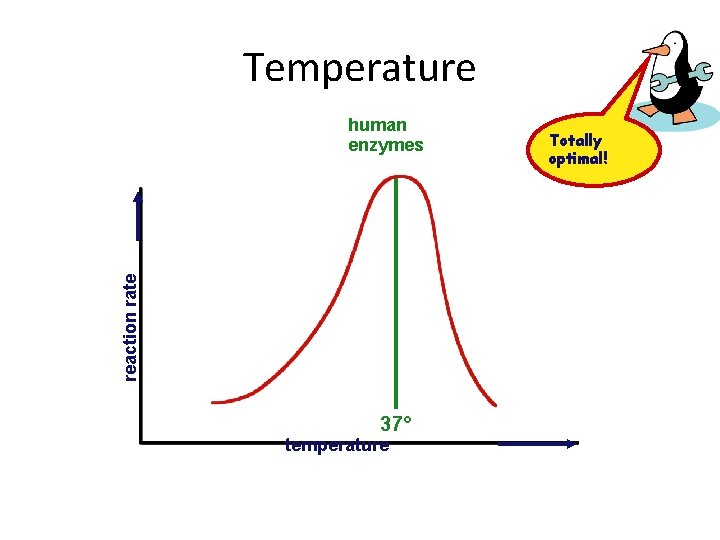
Temperature reaction rate human enzymes 37° temperature Totally optimal!

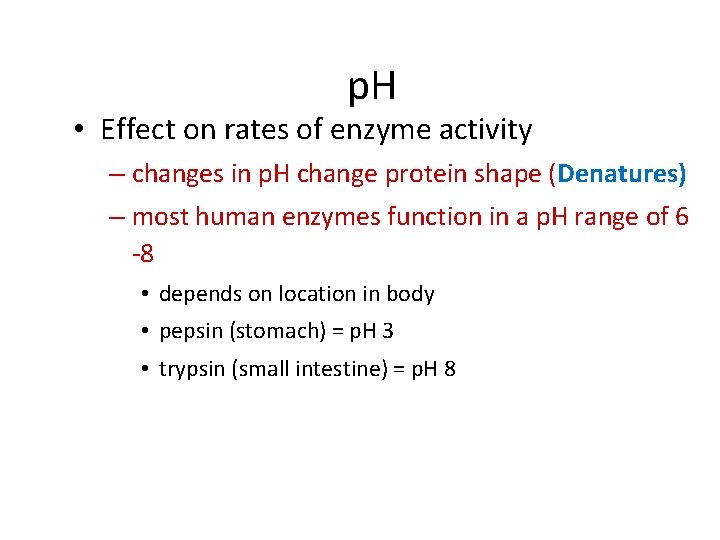
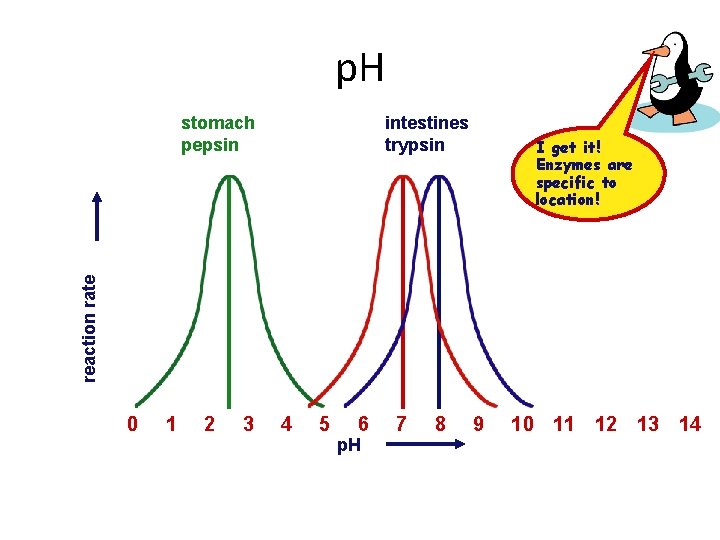
p. H • Effect on rates of enzyme activity – changes in p. H change protein shape (Denatures) – most human enzymes function in a p. H range of 6 -8 • depends on location in body • pepsin (stomach) = p. H 3 • trypsin (small intestine) = p. H 8

p. H intestines trypsin I get it! Enzymes are specific to location! reaction rate stomach pepsin 0 1 2 3 4 5 6 p. H 7 8 9 10 11 12 13 14

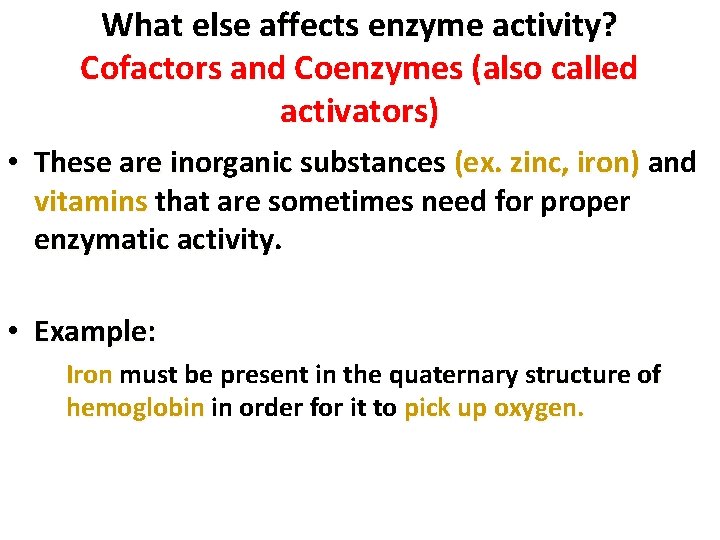
What else affects enzyme activity? Cofactors and Coenzymes (also called activators) • These are inorganic substances (ex. zinc, iron) and vitamins that are sometimes need for proper enzymatic activity • Example: Iron must be present in the quaternary structure of hemoglobin in order for it to pick up oxygen. 17

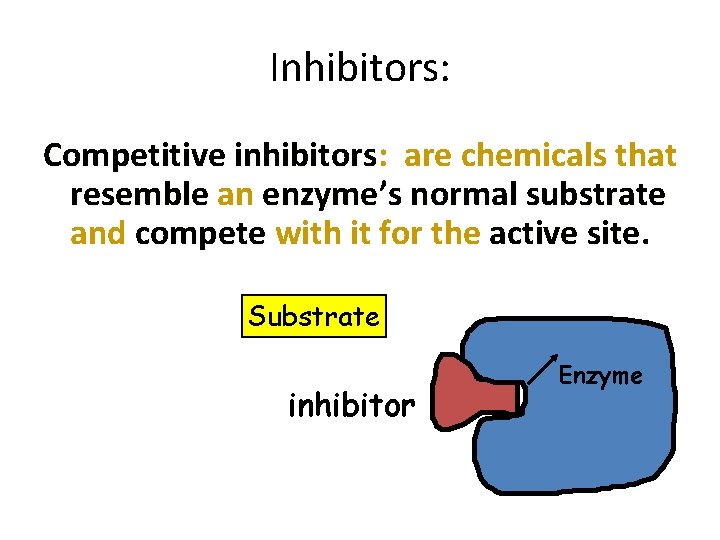
Inhibitors: Competitive inhibitors: are chemicals that resemble an enzyme’s normal substrate and compete with it for the active site Substrate Competitive inhibitor Enzyme 18

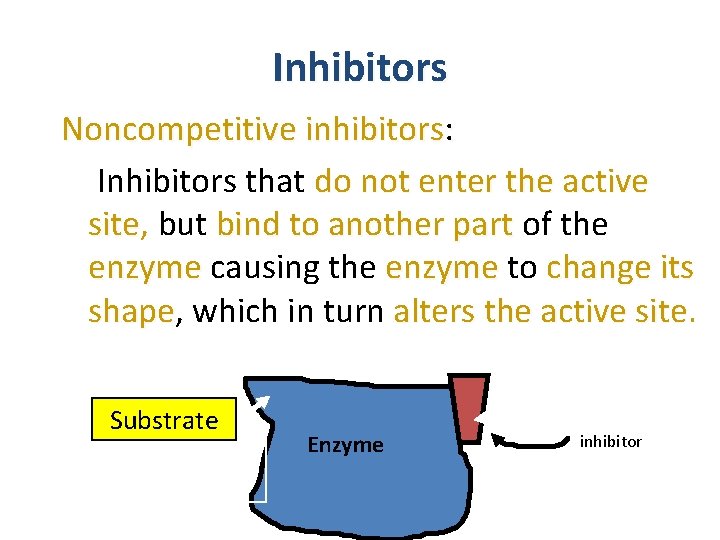
Inhibitors Noncompetitive inhibitors: Inhibitors that do not enter the active site, site but bind to another part of the enzyme causing the enzyme to change its shape, shape which in turn alters the active site Substrate active site altered Noncompetitive Inhibitor Enzyme inhibitor 19