Enzymes Chapter 8 Learning Targets I can explain









- Slides: 23

Enzymes Chapter 8

Learning Targets �I can explain how the change in the structure of a molecular system may result in a change of the function of the system.

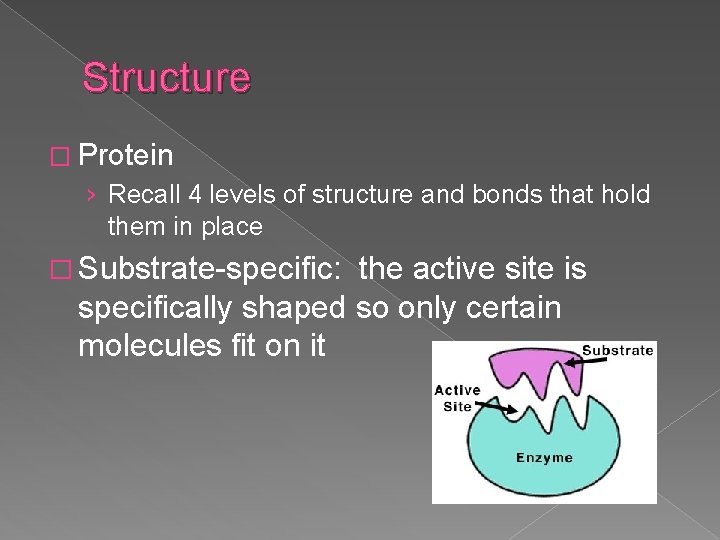
Structure � Protein › Recall 4 levels of structure and bonds that hold them in place � Substrate-specific: the active site is specifically shaped so only certain molecules fit on it

Learning Targets �I can explain how the shape of enzymes, active sites and interaction with specific molecules are essential for basic functioning of the enzyme. › I can explain how for an enzyme-mediated chemical reaction to occur, the substrate must be complementary to the surface properties (shape and charge of the active site). In other words, the substrate must fit into the enzyme’s active site.

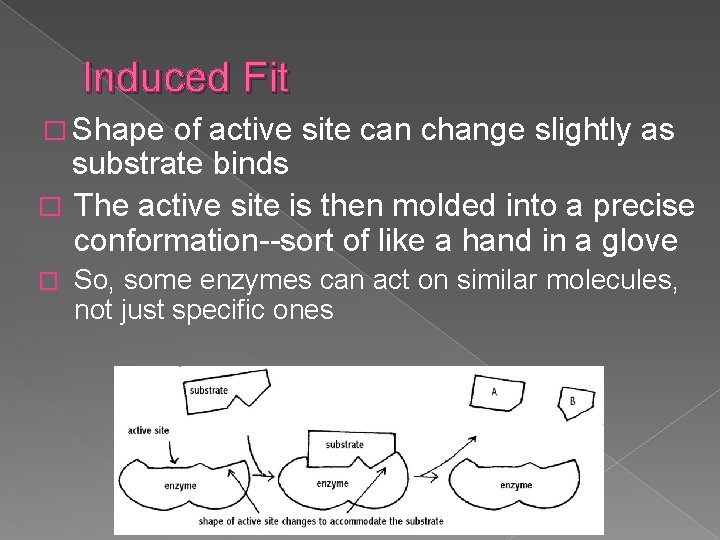
Induced Fit � Shape of active site can change slightly as substrate binds � The active site is then molded into a precise conformation--sort of like a hand in a glove � So, some enzymes can act on similar molecules, not just specific ones

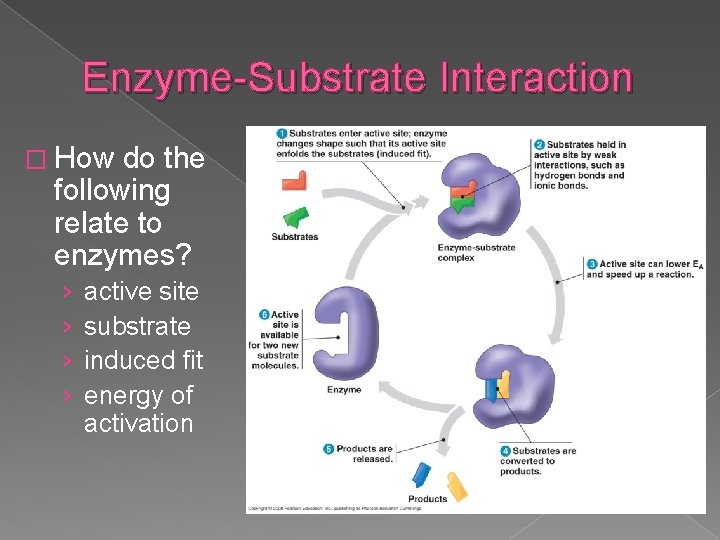
Enzyme-Substrate Interaction � How do the following relate to enzymes? › › active site substrate induced fit energy of activation

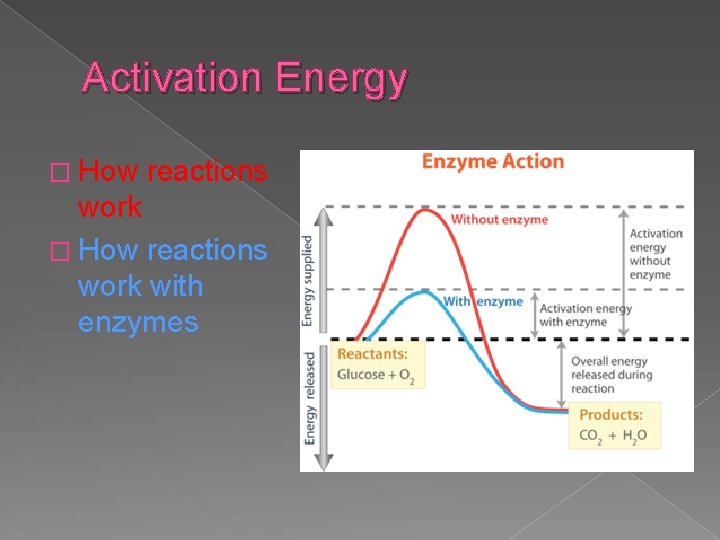
Activation Energy � How reactions work with enzymes

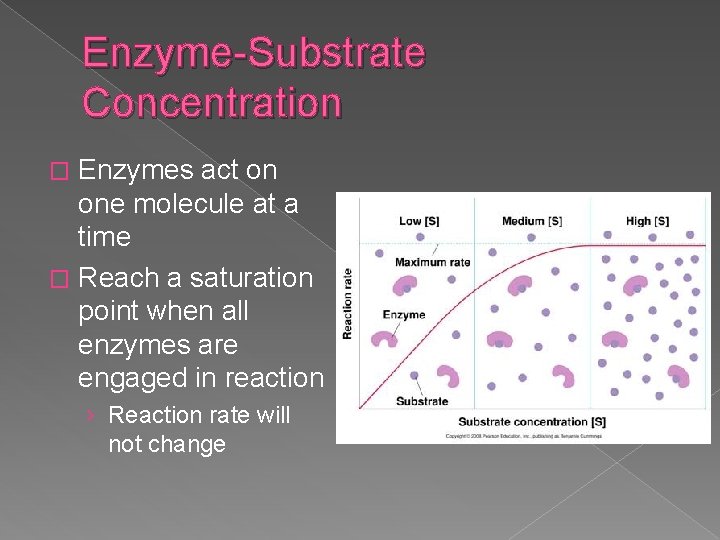
Enzyme-Substrate Concentration Enzymes act on one molecule at a time � Reach a saturation point when all enzymes are engaged in reaction � › Reaction rate will not change

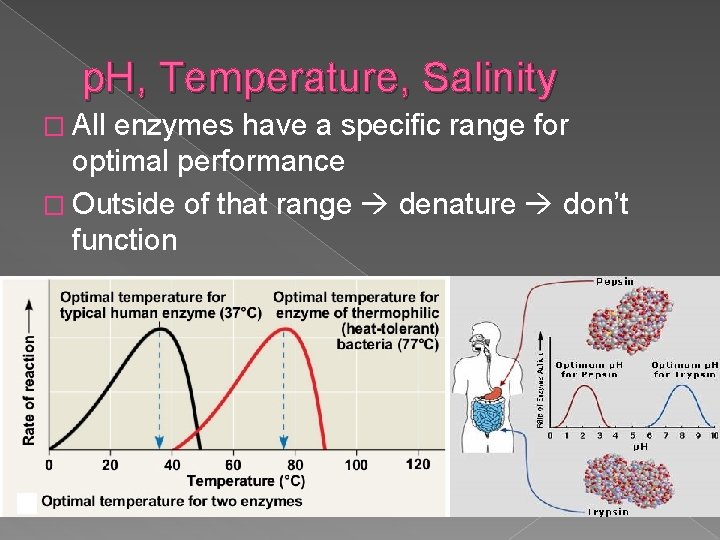
p. H, Temperature, Salinity � All enzymes have a specific range for optimal performance � Outside of that range denature don’t function

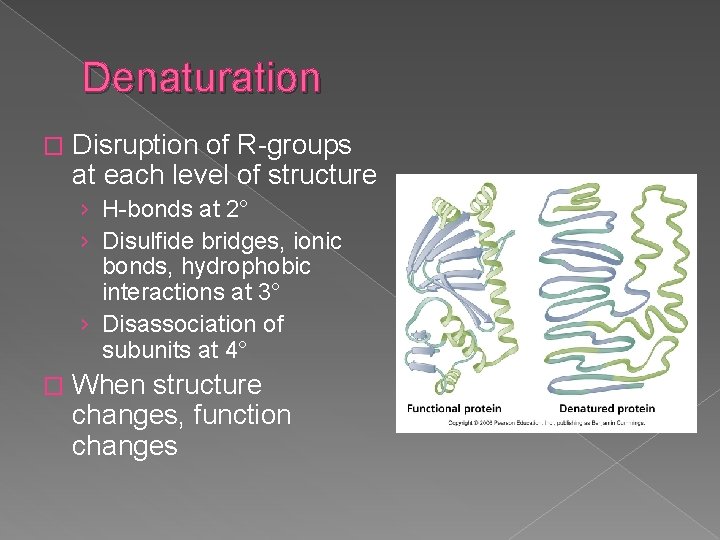
Denaturation � Disruption of R-groups at each level of structure › H-bonds at 2° › Disulfide bridges, ionic bonds, hydrophobic interactions at 3° › Disassociation of subunits at 4° � When structure changes, function changes

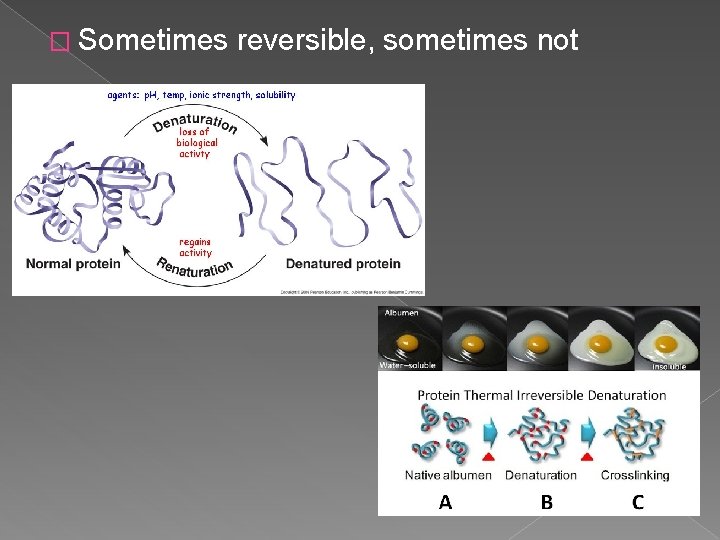
� Sometimes reversible, sometimes not

Learning Target › I can explain how cofactors and coenzymes affect enzyme function; this interaction relates to a structural change that alters the activity rate of the enzyme. The enzyme may only become active when all the appropriate cofactors or coenzymes are present and bind to the appropriate sites on the enzyme. › I can explain how other molecules and the environment in which the enzyme acts can enhance or inhibit enzyme activity. Molecules can bind reversibly or irreversibly to the active or allosteric sites, changing the activity of the enzyme.

Enhancing Factors Help/Increase Enzyme Function

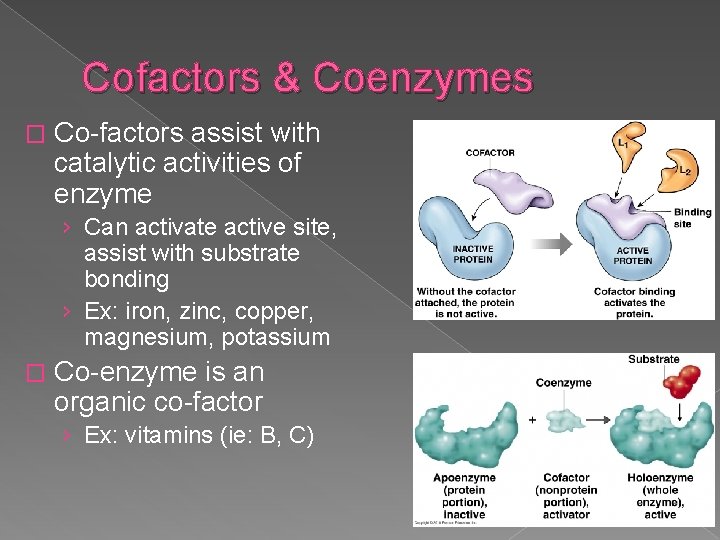
Cofactors & Coenzymes � Co-factors assist with catalytic activities of enzyme › Can activate active site, assist with substrate bonding › Ex: iron, zinc, copper, magnesium, potassium � Co-enzyme is an organic co-factor › Ex: vitamins (ie: B, C)

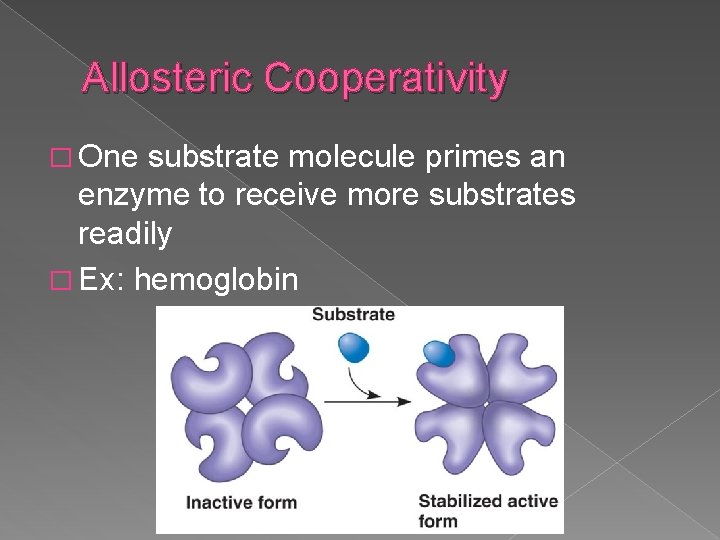
Allosteric Cooperativity � One substrate molecule primes an enzyme to receive more substrates readily � Ex: hemoglobin

Inhibiting Factors Prevent/Interfere with Enzyme Function

Competitive Inhibition � Competitive inhibitor has same shape as substrate � (competes for active site) � Prevents substrate from binding to active site by binding to active site itself

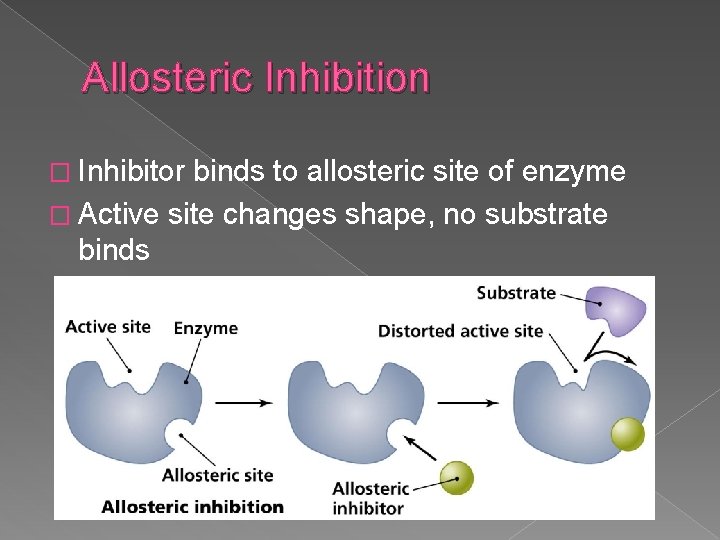
Allosteric Inhibition � Inhibitor binds to allosteric site of enzyme � Active site changes shape, no substrate binds

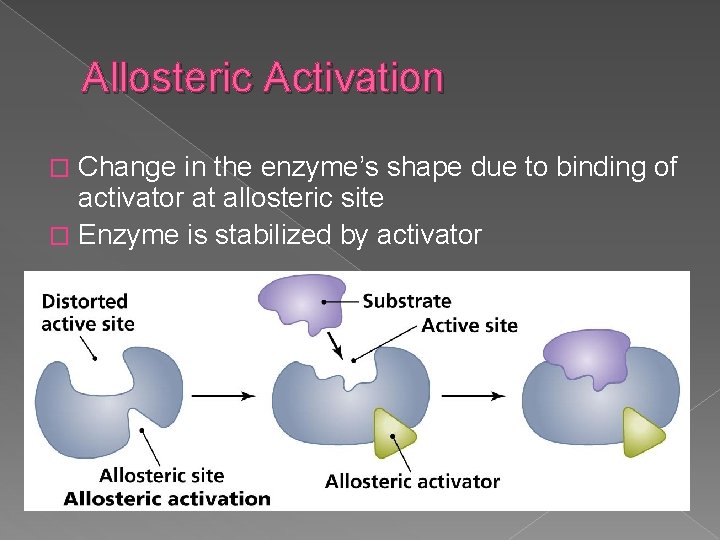
Allosteric Activation Change in the enzyme’s shape due to binding of activator at allosteric site � Enzyme is stabilized by activator �

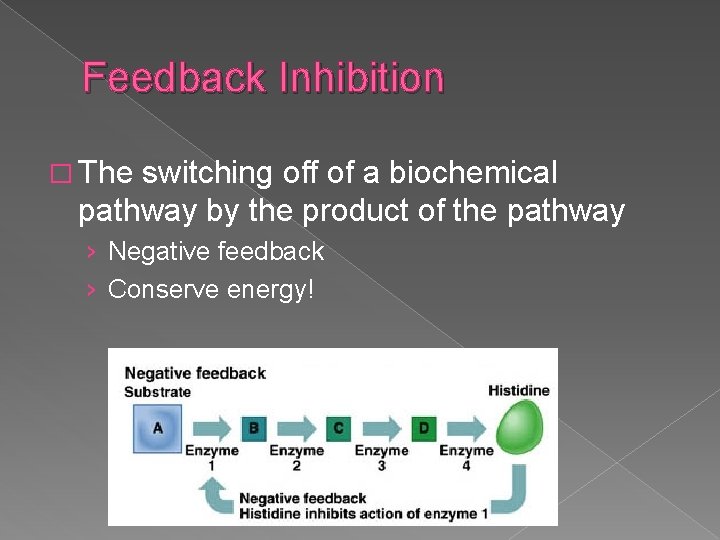
Feedback Inhibition � The switching off of a biochemical pathway by the product of the pathway › Negative feedback › Conserve energy!

� Bozeman Enzymes Video

Learning Targets � I can explain that the change in function of an enzyme can be interpreted from data regarding the concentrations of product or substrate as a function of time. These representations demonstrate the relationship between an enzyme’s activity, the disappearance of substrate, and/or presence of a competitive inhibitor.

AP Lab 13 � Bozeman video