Enzymes AP Biology General Information l Globular proteins

Enzymes AP Biology
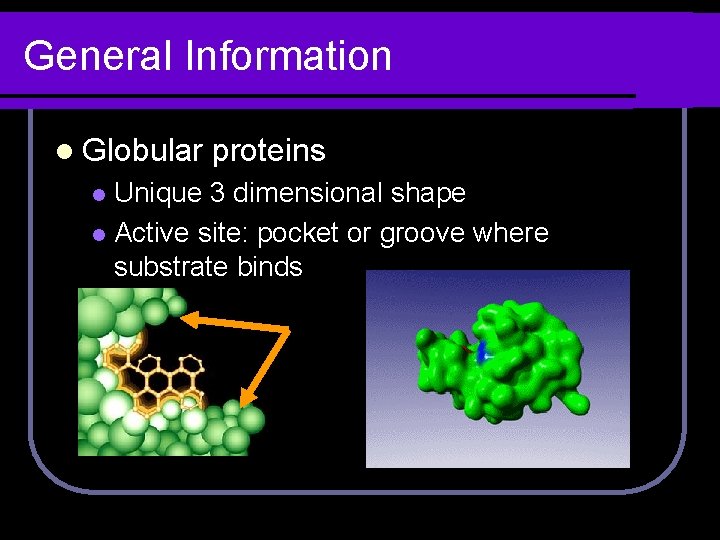
General Information l Globular proteins Unique 3 dimensional shape l Active site: pocket or groove where substrate binds l
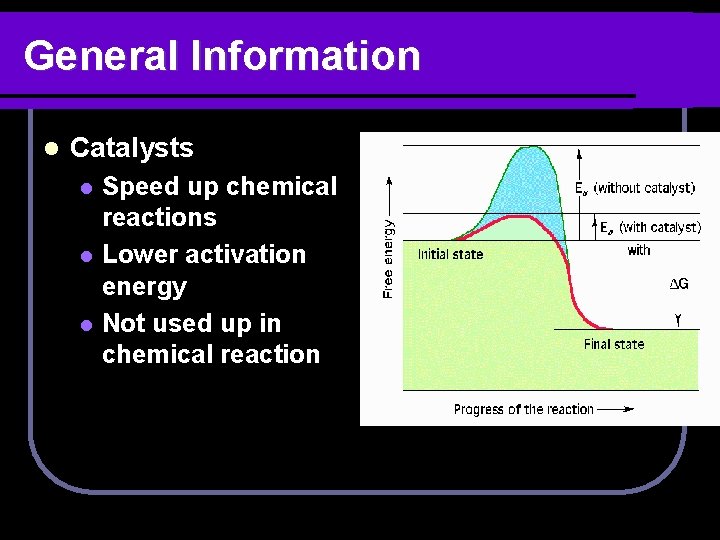
General Information l Catalysts l l l Speed up chemical reactions Lower activation energy Not used up in chemical reaction
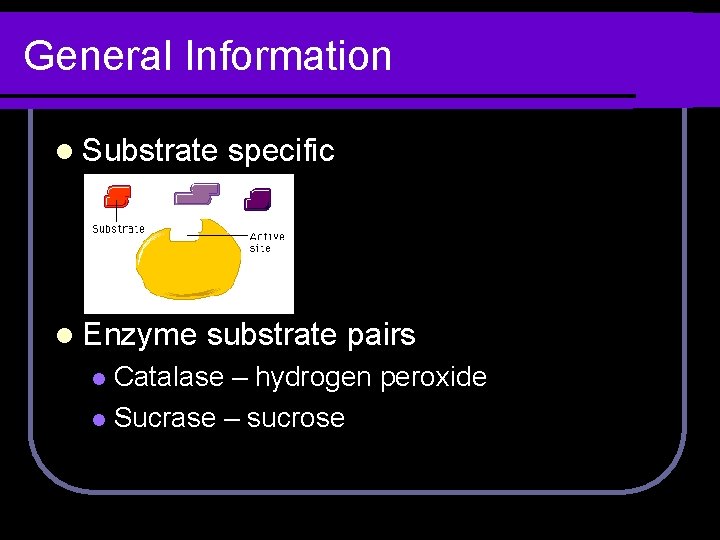
General Information l Substrate l Enzyme specific substrate pairs Catalase – hydrogen peroxide l Sucrase – sucrose l
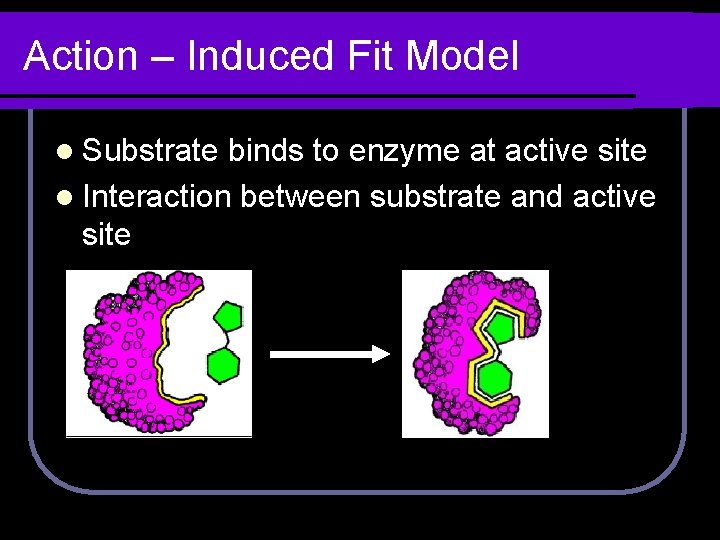
Action – Induced Fit Model l Substrate binds to enzyme at active site l Interaction between substrate and active site
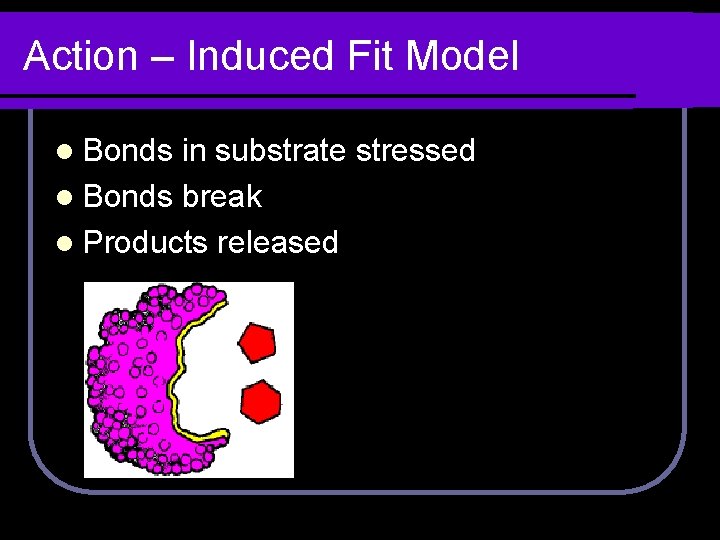
Action – Induced Fit Model l Bonds in substrate stressed l Bonds break l Products released
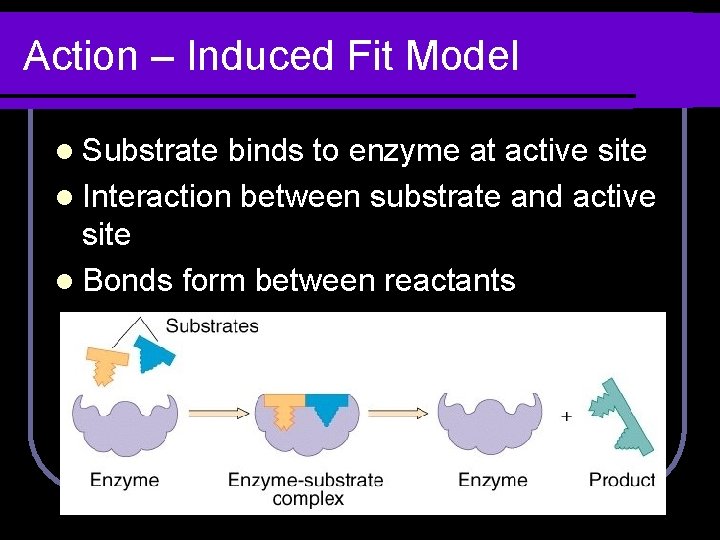
Action – Induced Fit Model l Substrate binds to enzyme at active site l Interaction between substrate and active site l Bonds form between reactants
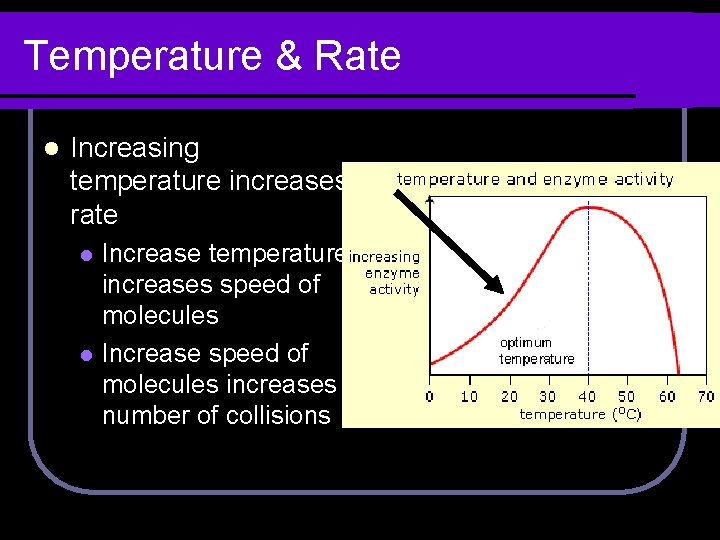
Temperature & Rate l Increasing temperature increases rate l l Increase temperature increases speed of molecules Increase speed of molecules increases number of collisions
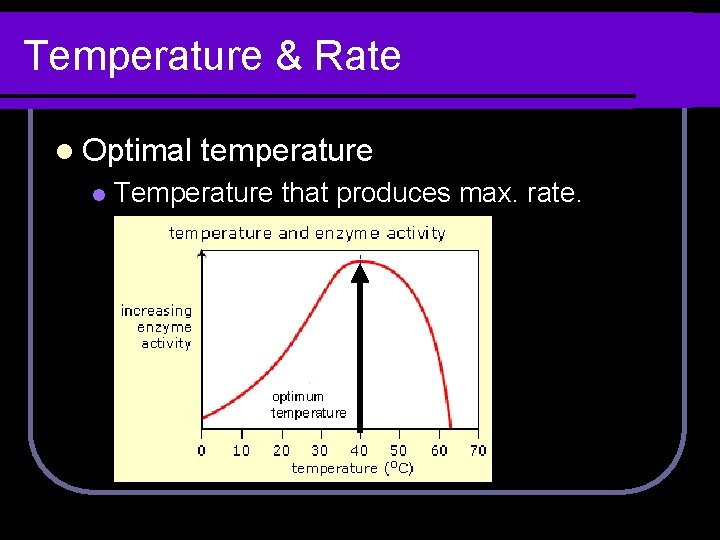
Temperature & Rate l Optimal l temperature Temperature that produces max. rate.
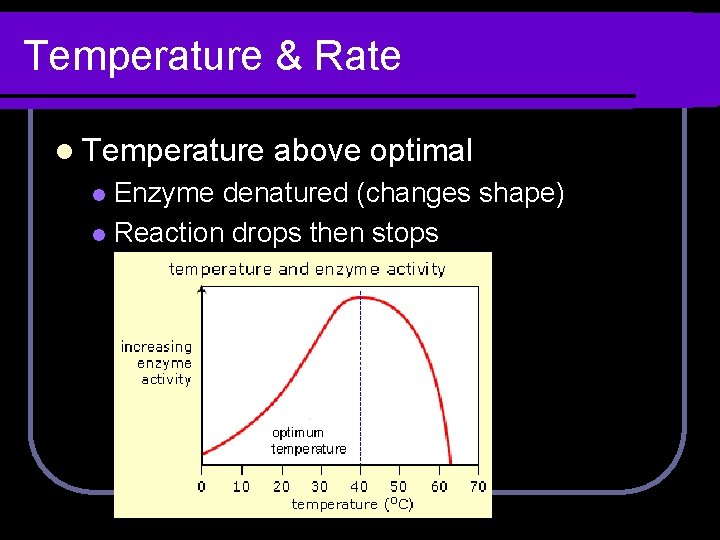
Temperature & Rate l Temperature above optimal Enzyme denatured (changes shape) l Reaction drops then stops l
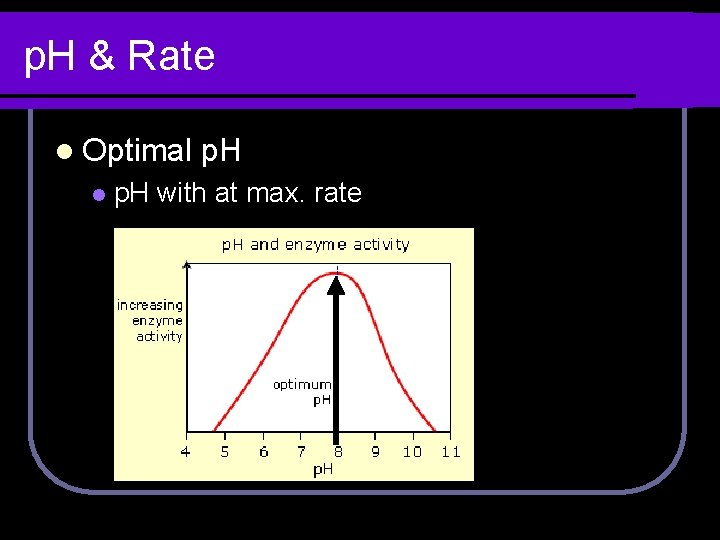
p. H & Rate l Optimal l p. H with at max. rate
![p. H & Rate l Above optimal – rate decreases l Lower [H+] interferes p. H & Rate l Above optimal – rate decreases l Lower [H+] interferes](http://slidetodoc.com/presentation_image_h2/3031d2b2fb963657c3027a9d6c420e6b/image-12.jpg)
p. H & Rate l Above optimal – rate decreases l Lower [H+] interferes with enzyme shape
![p. H & Rate l Below optimal – rate decreases l Higher [H+] interferes p. H & Rate l Below optimal – rate decreases l Higher [H+] interferes](http://slidetodoc.com/presentation_image_h2/3031d2b2fb963657c3027a9d6c420e6b/image-13.jpg)
p. H & Rate l Below optimal – rate decreases l Higher [H+] interferes with enzyme shape
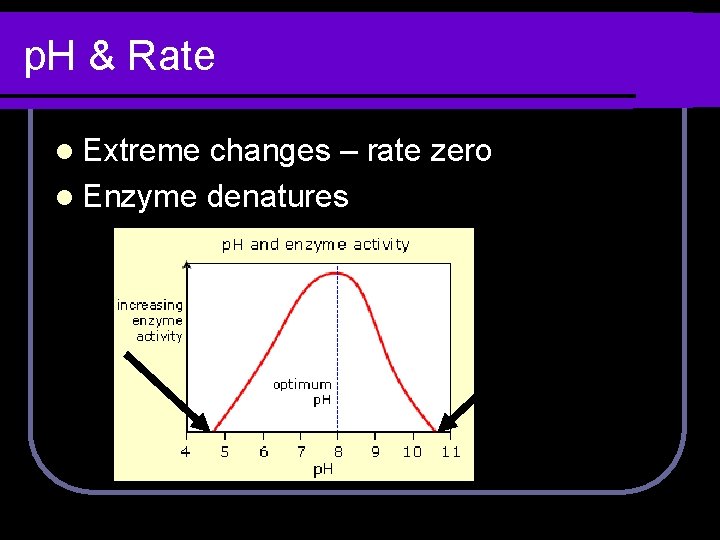
p. H & Rate l Extreme changes – rate zero l Enzyme denatures
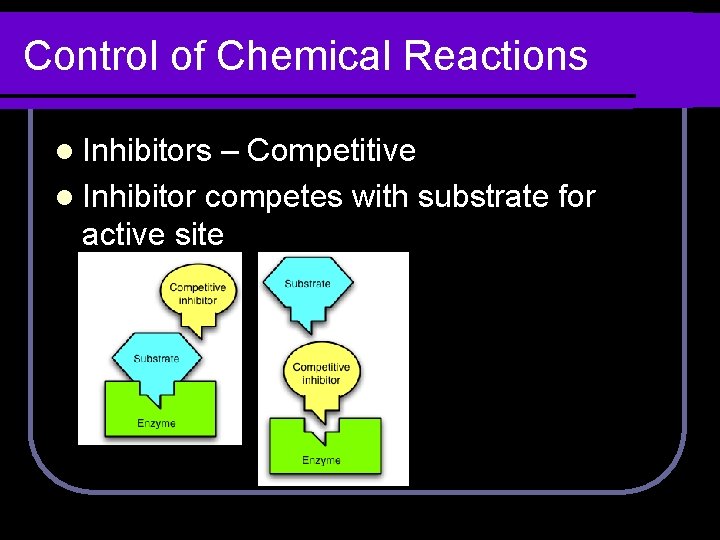
Control of Chemical Reactions l Inhibitors – Competitive l Inhibitor competes with substrate for active site
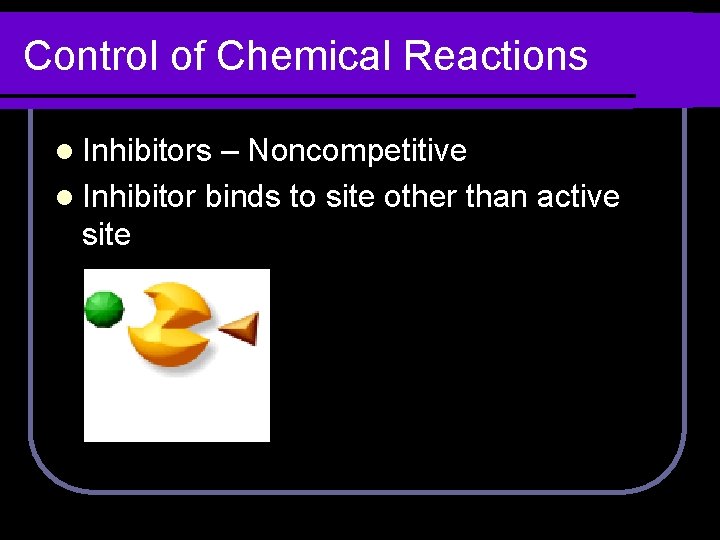
Control of Chemical Reactions l Inhibitors – Noncompetitive l Inhibitor binds to site other than active site
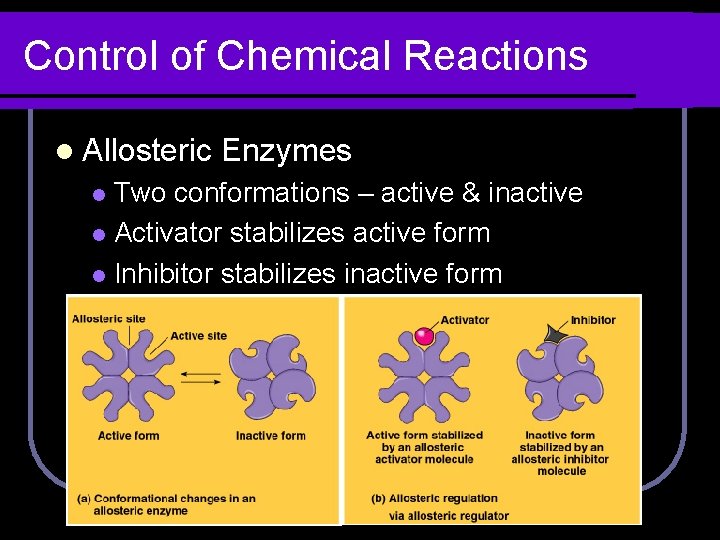
Control of Chemical Reactions l Allosteric Enzymes Two conformations – active & inactive l Activator stabilizes active form l Inhibitor stabilizes inactive form l
- Slides: 17