Enzyme Units Expressed in terms of the activity





- Slides: 21

Enzyme Units • Expressed in terms of the activity • One International Unit- the amount of Enzyme that catalyzes the formation of 1 micromole of product in 1 minute • Katal- amount of enzyme catalyzing the conversion of 1 mole of substrate to product in 1 second • 1 katal = 6 X 107 international units

Enzyme Inhibition/ Inhibitor • Inhibition- The decrease in enzyme activity • Inhibitors -substances that decrease the catalytic activity of enzymes • May be protein/ Non protein • According to mode of action- classify in 2 categories 1. Reversible 2. Irreversible

Reversible Inhibition • Binds non covalently • Can be reversed if inhibitor is removed • Classify into 1. Competitive 2. Non- competitive 3. Un competitive

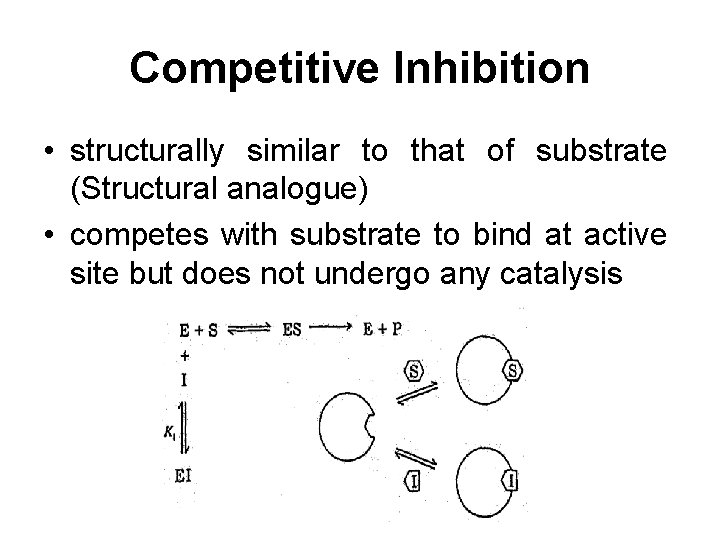
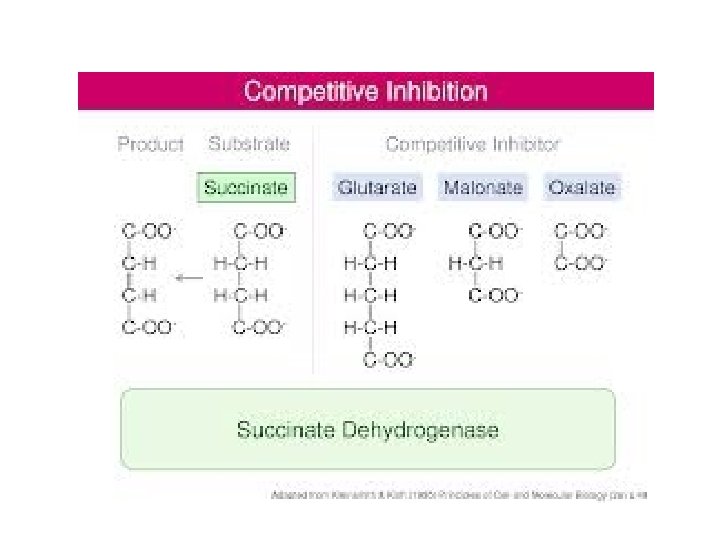
Competitive Inhibition • structurally similar to that of substrate (Structural analogue) • competes with substrate to bind at active site but does not undergo any catalysis

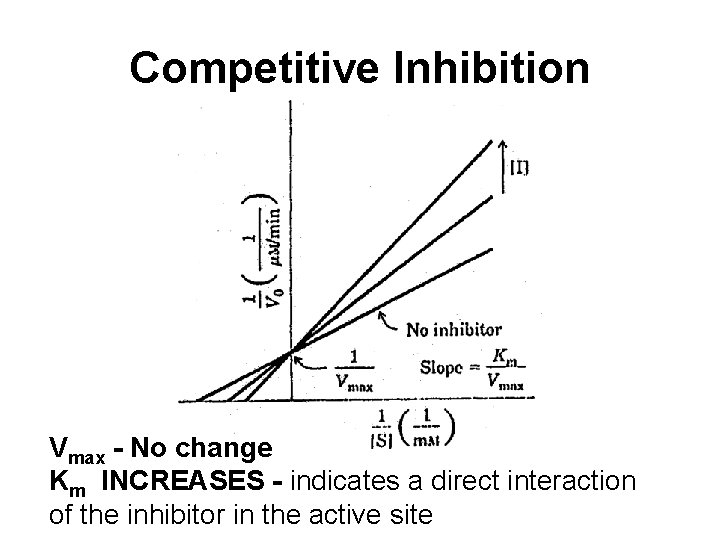
Competitive Inhibition Vmax - No change Km INCREASES - indicates a direct interaction of the inhibitor in the active site

• increasing the amount of substrate can overcome the effect of the inhibitor as number of enzyme molecules available for the inhibitor are far less, and restore the normal rate of the enzyme catalyzed reaction (Reversible) • e. g. succinate dehydrogenase- Malonate competitively inhibits the enzyme because it is structurally similar to the substrate succinate


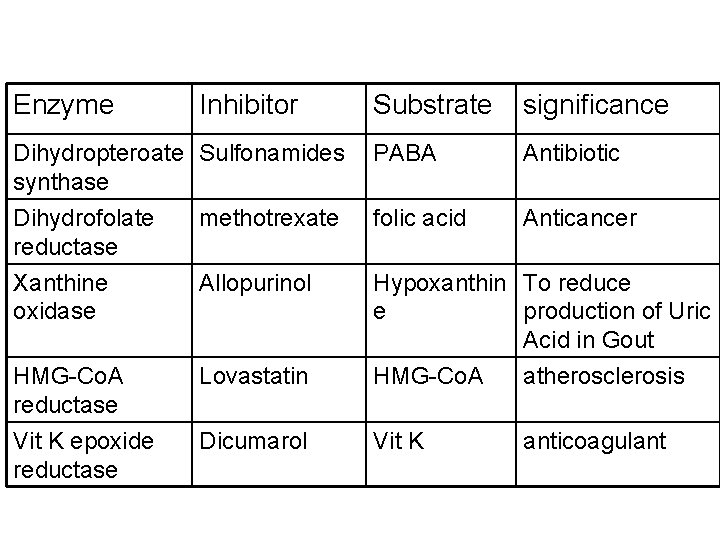
Competitive Inhibitors as Chemotherapeutic Agents • in clinical situations, the competitive inhibitors are called as antagonists or anti metabolites of the substrate with which they compete • Competitive inhibitors are useful chemotherapeutic agents used as 1. Antibiotics 2. Anti-cancer drugs 3. In the treatment of metabolic diseases like gout, atherosclerosis and hypertension

Enzyme Inhibitor Substrate significance Dihydropteroate Sulfonamides synthase PABA Antibiotic Dihydrofolate reductase Xanthine oxidase methotrexate folic acid Anticancer Allopurinol Hypoxanthin To reduce e production of Uric Acid in Gout HMG-Co. A reductase Lovastatin HMG-Co. A atherosclerosis Vit K epoxide reductase Dicumarol Vit K anticoagulant

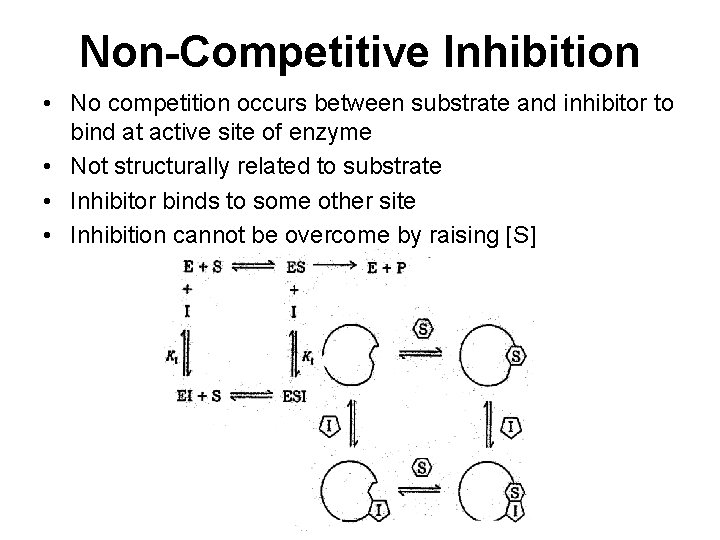
Non-Competitive Inhibition • No competition occurs between substrate and inhibitor to bind at active site of enzyme • Not structurally related to substrate • Inhibitor binds to some other site • Inhibition cannot be overcome by raising [S]

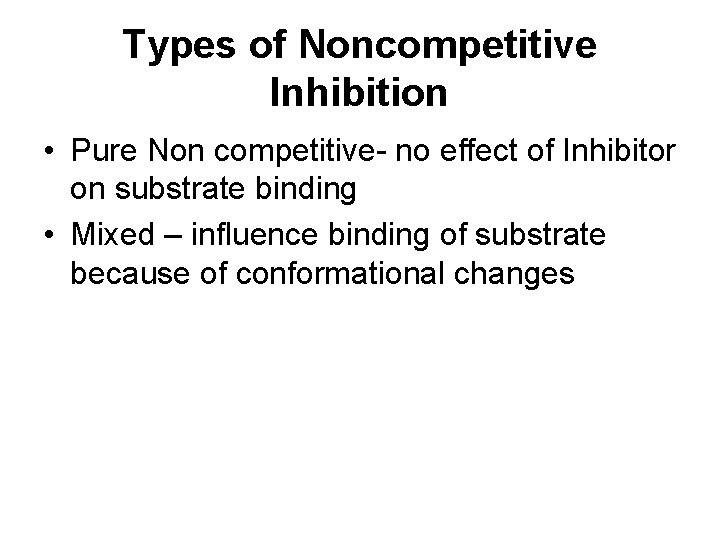
Types of Noncompetitive Inhibition • Pure Non competitive- no effect of Inhibitor on substrate binding • Mixed – influence binding of substrate because of conformational changes

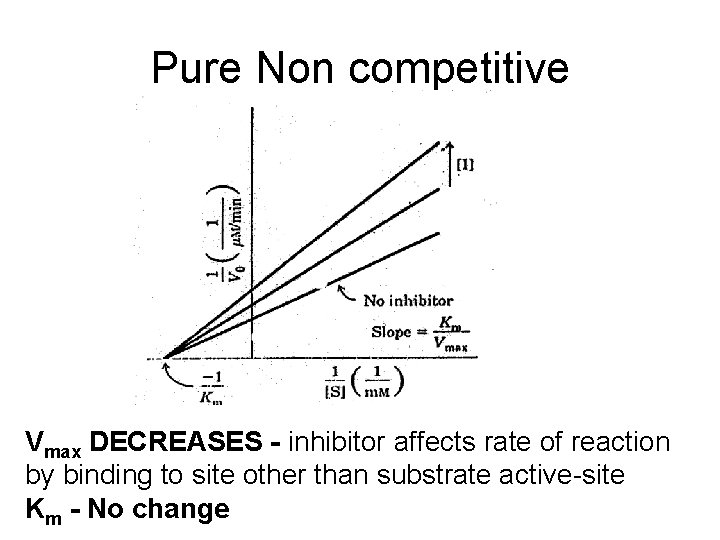
Pure Non competitive Vmax DECREASES - inhibitor affects rate of reaction by binding to site other than substrate active-site Km - No change

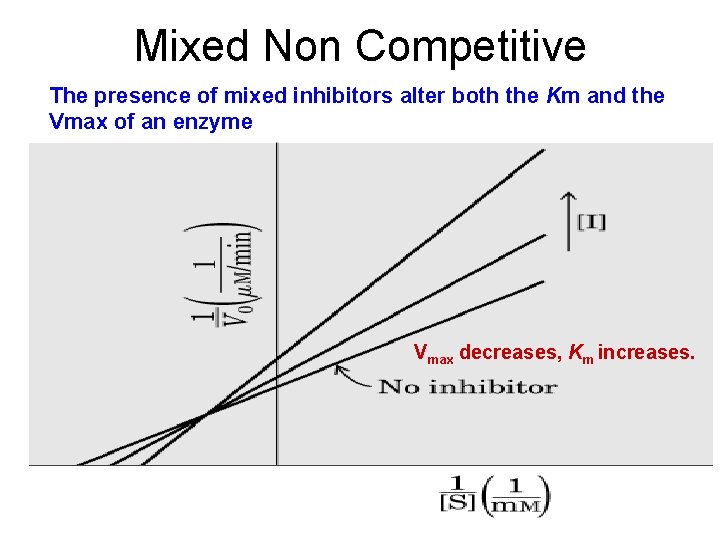
Mixed Non Competitive The presence of mixed inhibitors alter both the Km and the Vmax of an enzyme Vmax decreases, Km increases.

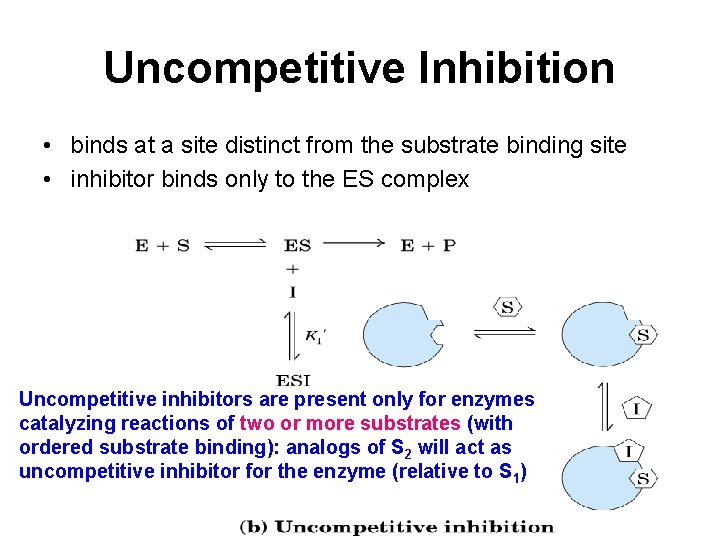
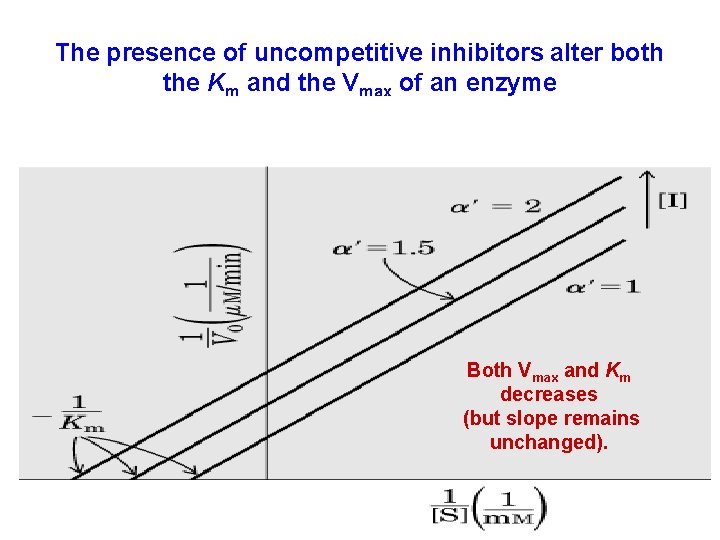
Uncompetitive Inhibition • binds at a site distinct from the substrate binding site • inhibitor binds only to the ES complex Uncompetitive inhibitors are present only for enzymes catalyzing reactions of two or more substrates (with ordered substrate binding): analogs of S 2 will act as uncompetitive inhibitor for the enzyme (relative to S 1)

The presence of uncompetitive inhibitors alter both the Km and the Vmax of an enzyme Both Vmax and Km decreases (but slope remains unchanged).

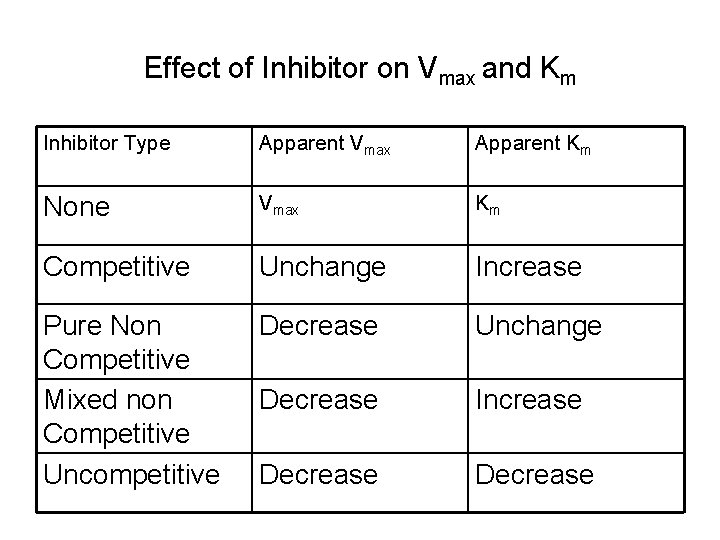
Effect of Inhibitor on Vmax and Km Inhibitor Type Apparent Vmax Apparent Km None Vmax Km Competitive Unchange Increase Pure Non Competitive Mixed non Competitive Uncompetitive Decrease Unchange Decrease Increase Decrease

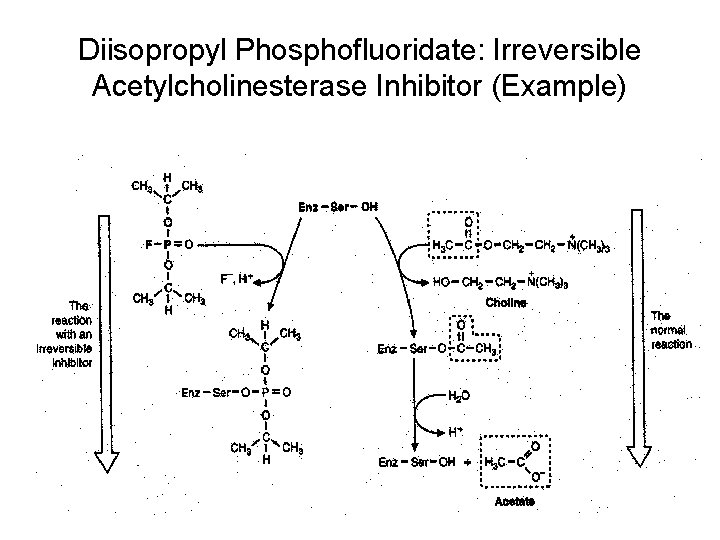
Irrversible Inhibitor • Chemically modify or form tight noncovalent interactions with functional groups in the active site of enzymes • Net effect is a loss of active enzyme • Dilution or dialysis of the enzyme-inhibitor solution does not dissociate the EI complex so no restoration of enzyme activity

• Covalent bonds take longer time to form so irreversible inhibition is a timedependent process, with more enzyme being inactivated with increasing time e. g. Iodoacetateglyceraldehyde 3 phosphate dehydrogenase

Diisopropyl Phosphofluoridate: Irreversible Acetylcholinesterase Inhibitor (Example)

Suicide inhibitor • Mechanism-based inactivator • Inhibitory substrate analogs designed so that, via normal catalytic action of the enzyme, a very reactive group is generated and forms a covalent bond with a nearby functional group within the active site of the enzyme causing irreversible inhibition e. g. Penicillin

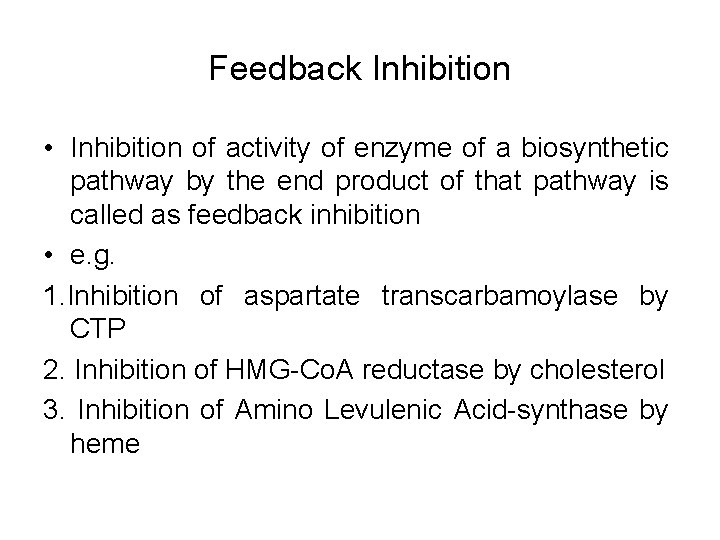
Feedback Inhibition • Inhibition of activity of enzyme of a biosynthetic pathway by the end product of that pathway is called as feedback inhibition • e. g. 1. Inhibition of aspartate transcarbamoylase by CTP 2. Inhibition of HMG-Co. A reductase by cholesterol 3. Inhibition of Amino Levulenic Acid-synthase by heme