enzyme substrate E S enzymesubstrate complex ES enzymesubstrate





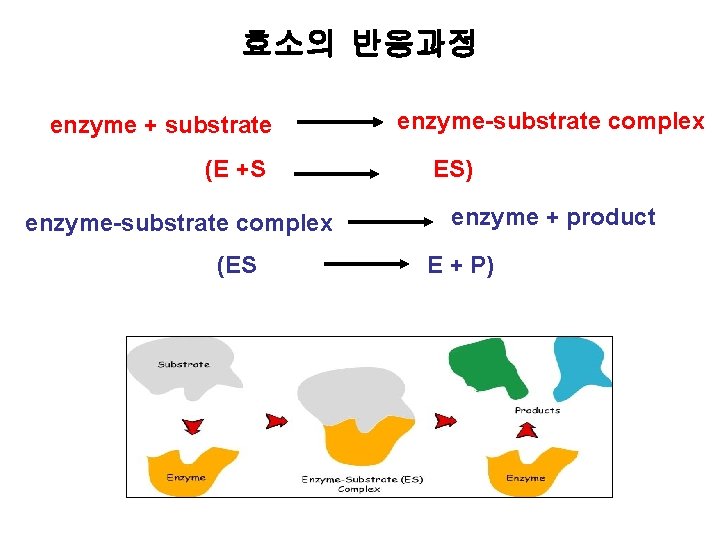
효소의 반응과정 enzyme + substrate (E +S enzyme-substrate complex (ES enzyme-substrate complex ES) enzyme + product E + P)
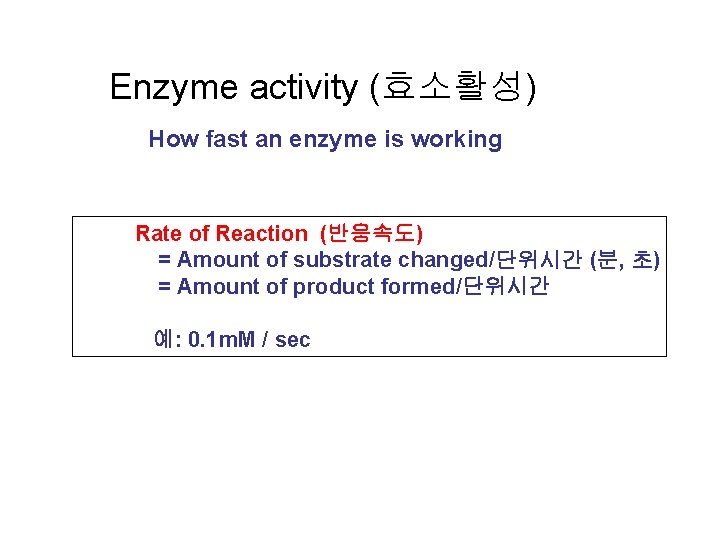
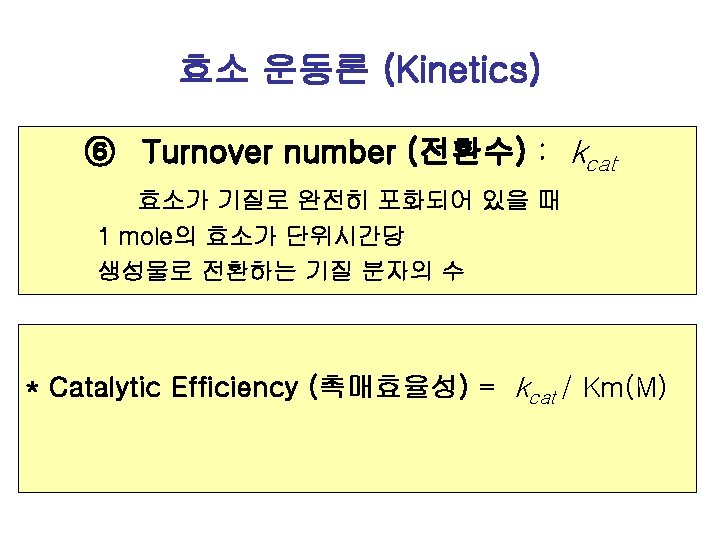
Enzyme activity (효소활성) How fast an enzyme is working Rate of Reaction (반응속도) = Amount of substrate changed/단위시간 (분, 초) = Amount of product formed/단위시간 예: 0. 1 m. M / sec




④ 분해 효소 (lyase) • an enzyme that catalyzes the breaking (an "elimination" reaction) of various chemical bonds by means other than hydrolysis and oxidation. • often forming a new double bond or a new ring structure. • ATP → c. AMP + Ppi • Ex) adenylate cyclase
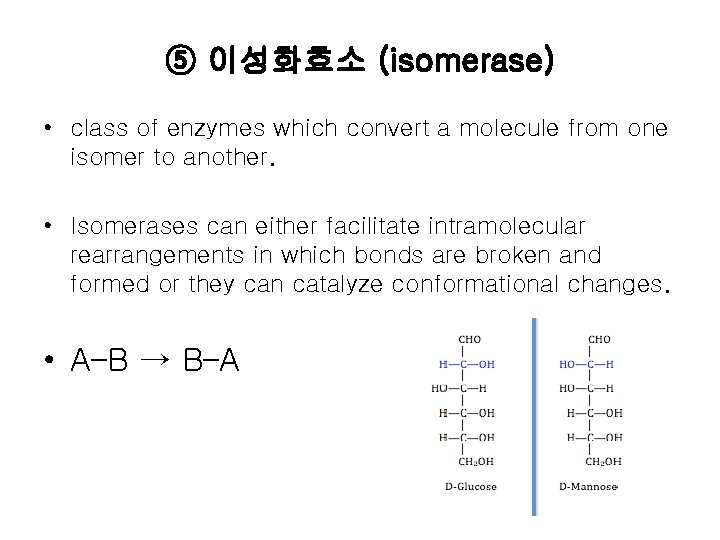
⑤ 이성화효소 (isomerase) • class of enzymes which convert a molecule from one isomer to another. • Isomerases can either facilitate intramolecular rearrangements in which bonds are broken and formed or they can catalyze conformational changes. • A–B → B–A

⑥ 합성효소 (synthase/synthetase, ligase) • an enzyme that can catalyze the joining of two large molecules by forming a new chemical bond, usually with accompanying hydrolysis of a small chemical group • the enzyme catalyzing the linking together of two compounds • Ab + C → A–C + b • Ab + c. D → A–D + b + c • Ex) DNA ligase • Synthases (do not use energy from nucleoside tirphosphates suh as ATP, GTP. . ) • Synthetases (use nucleoside triphosphates)
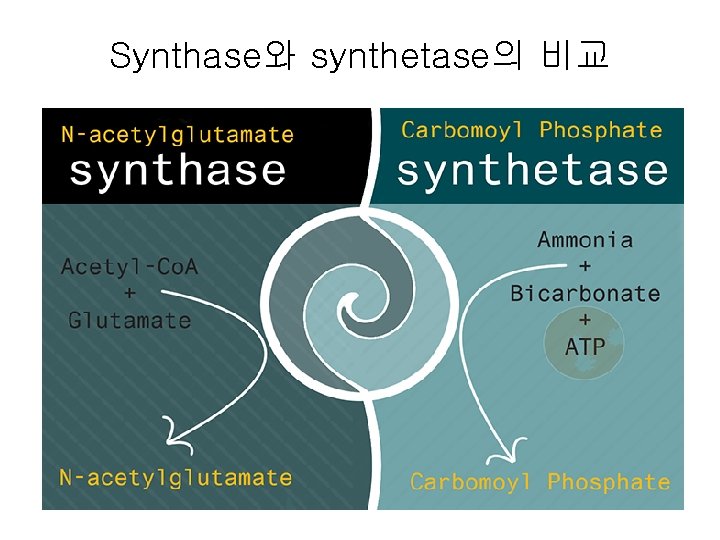
Synthase와 synthetase의 비교
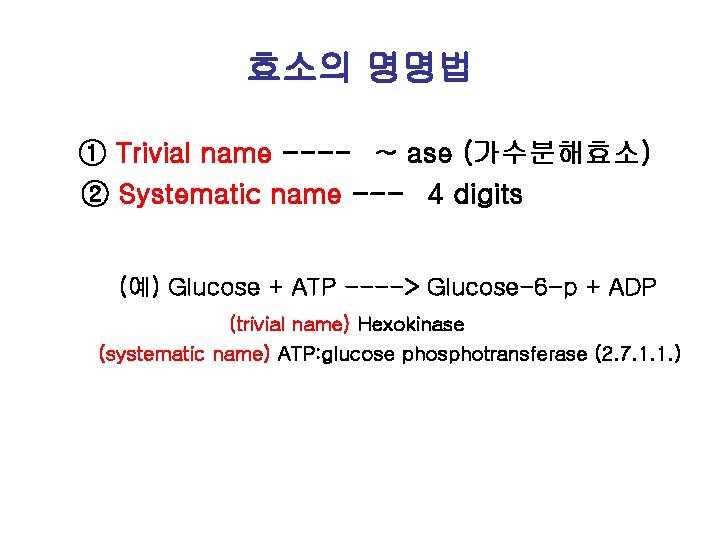
효소의 명명법 ① Trivial name ---- ~ ase (가수분해효소) ② Systematic name --- 4 digits (예) Glucose + ATP ----> Glucose-6 -p + ADP (trivial name) Hexokinase (systematic name) ATP: glucose phosphotransferase (2. 7. 1. 1. )

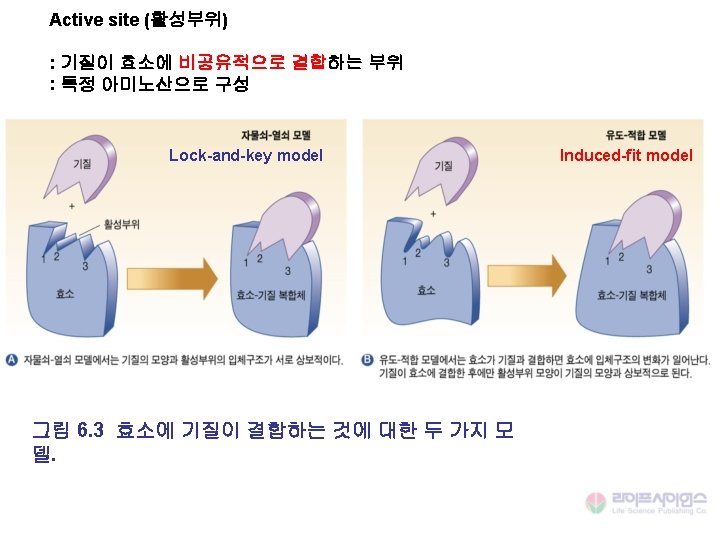
Interactions for Binding 1. Hydrogen bonds 2. Salt links 3. Van der Waals interactions 4. Hydrophobic effect
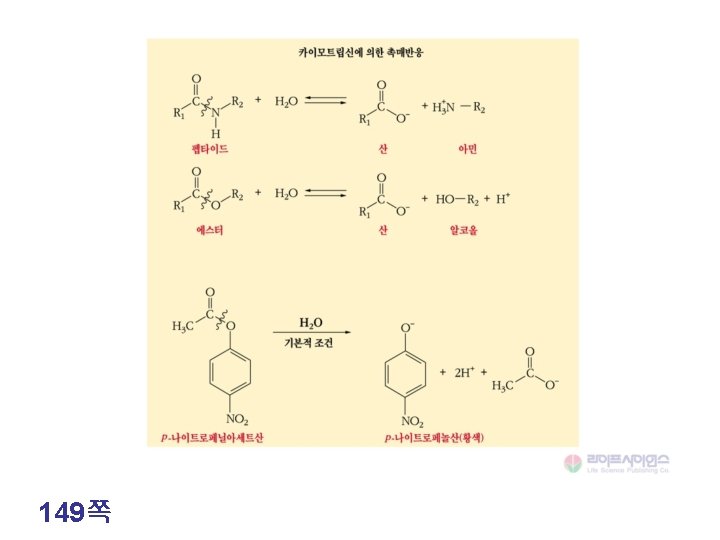
149쪽


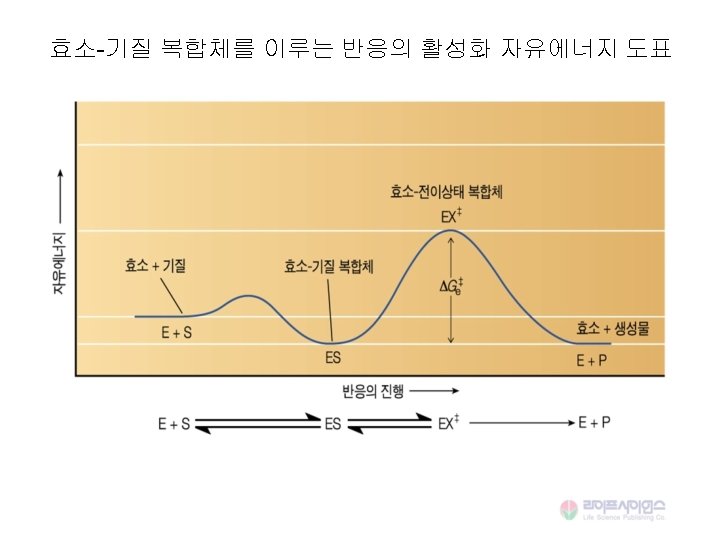
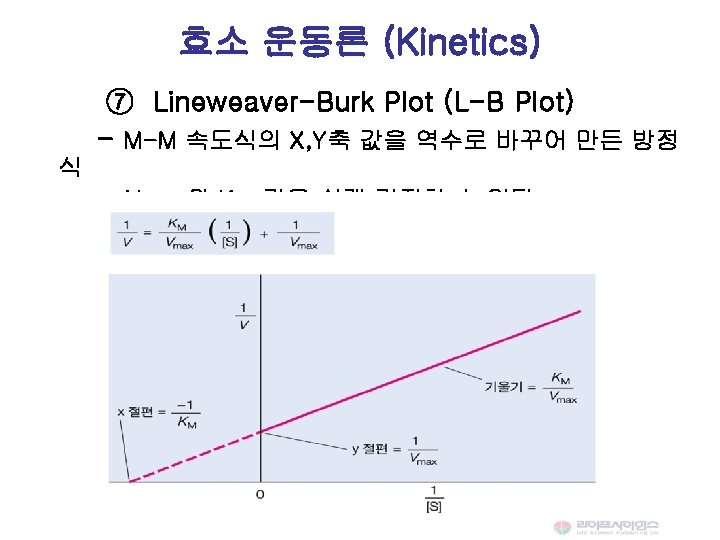
![Michaelis-Menten 속도식 Vmax [S] V = -------Km + [S] When [S] <<<Km, V = Michaelis-Menten 속도식 Vmax [S] V = -------Km + [S] When [S] <<<Km, V =](http://slidetodoc.com/presentation_image_h2/89789599a683850968488f64fce3231f/image-33.jpg)
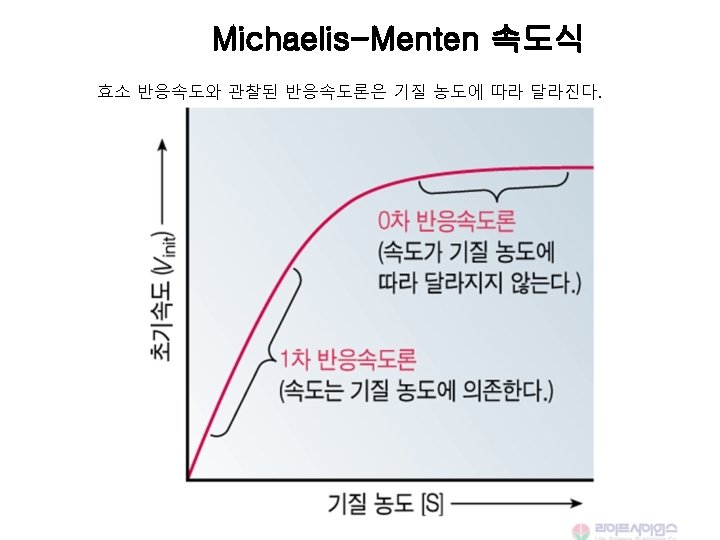
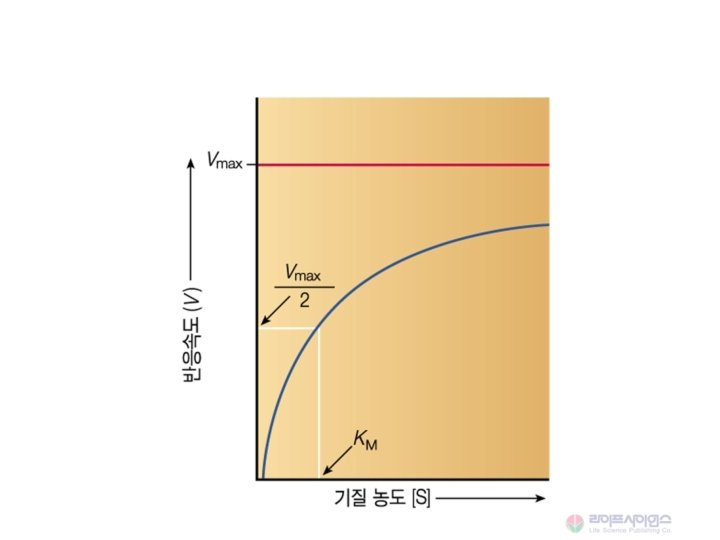
Michaelis-Menten 속도식 Vmax [S] V = -------Km + [S] When [S] <<<Km, V = Vmax [S] / Km When [S] =Km, V = Vmax / 2 When [S] >> Km, V = Vmax
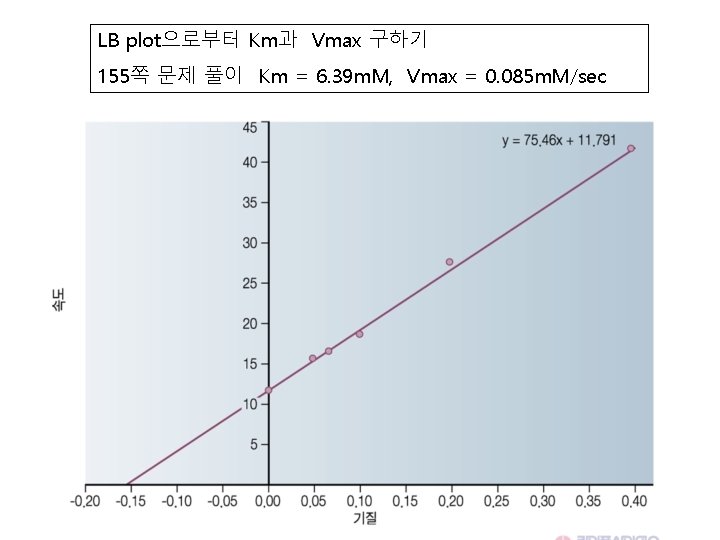
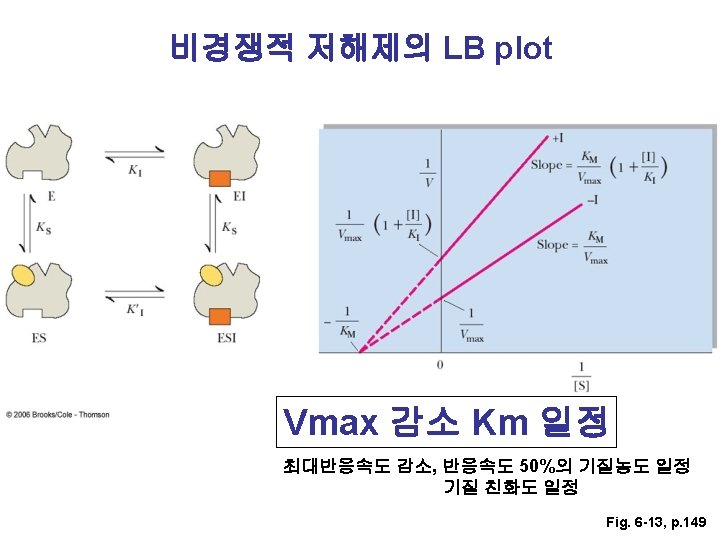
LB plot으로부터 Km과 Vmax 구하기 155쪽 문제 풀이 Km = 6. 39 m. M, Vmax = 0. 085 m. M/sec

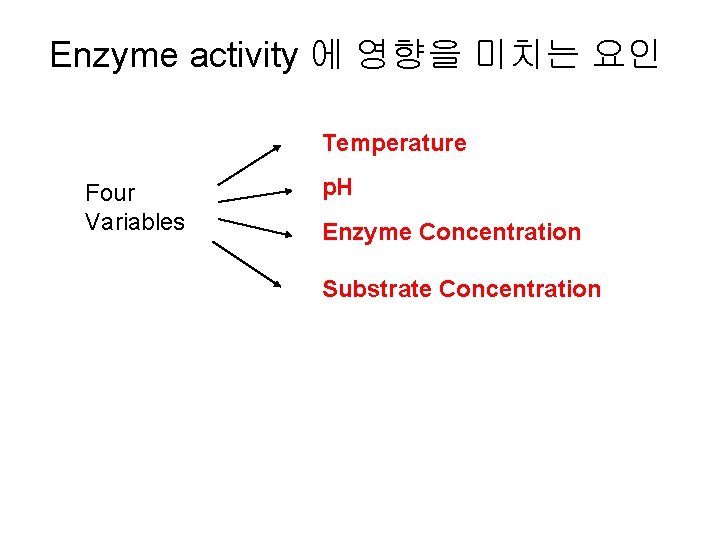
Enzyme activity 에 영향을 미치는 요인 Temperature Four Variables p. H Enzyme Concentration Substrate Concentration
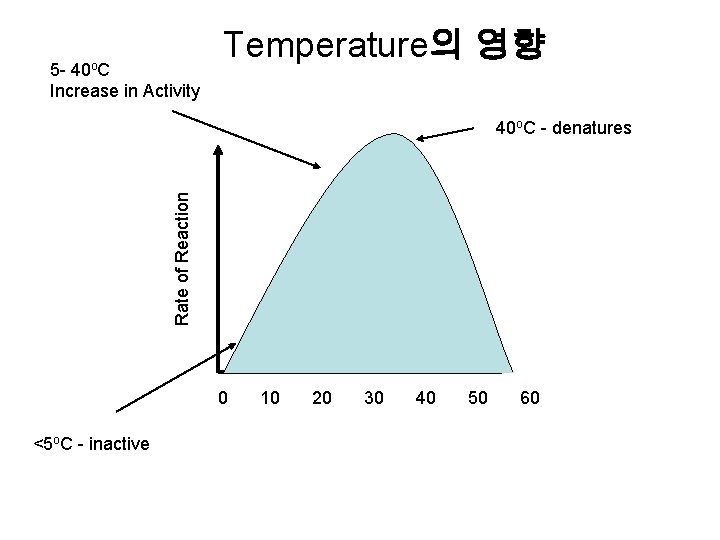
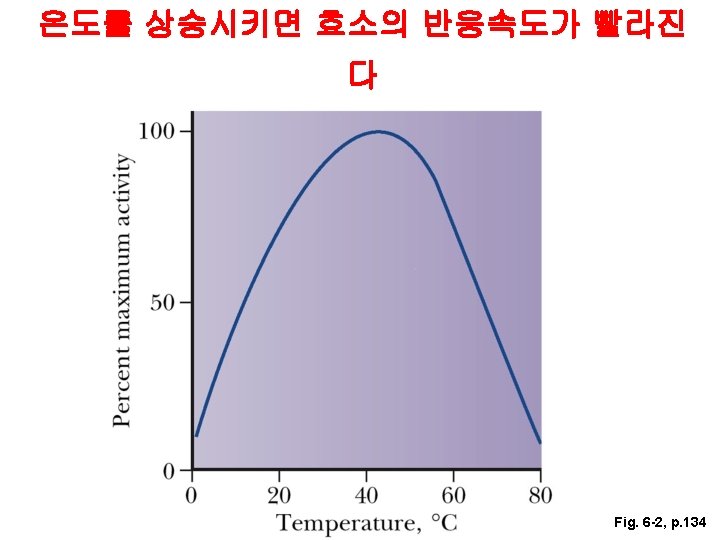
40 o. C 5 Increase in Activity Temperature의 영향 Rate of Reaction 40 o. C - denatures 0 <5 o. C - inactive 10 20 30 40 50 60
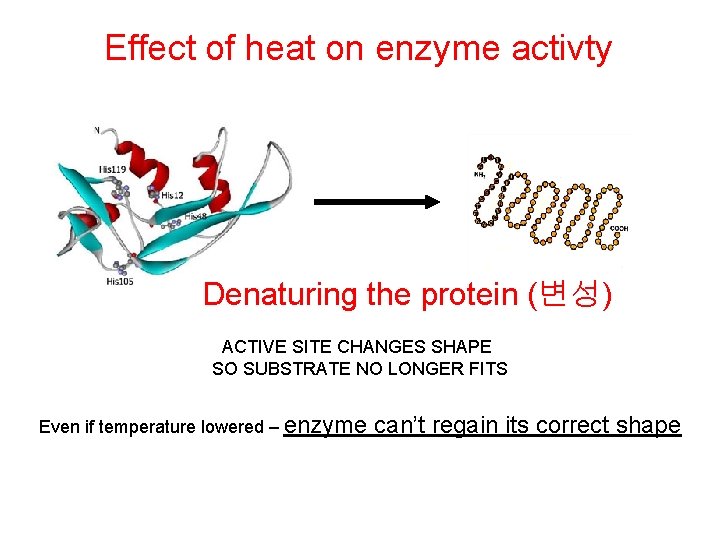
Effect of heat on enzyme activty Denaturing the protein (변성) ACTIVE SITE CHANGES SHAPE SO SUBSTRATE NO LONGER FITS Even if temperature lowered – enzyme can’t regain its correct shape
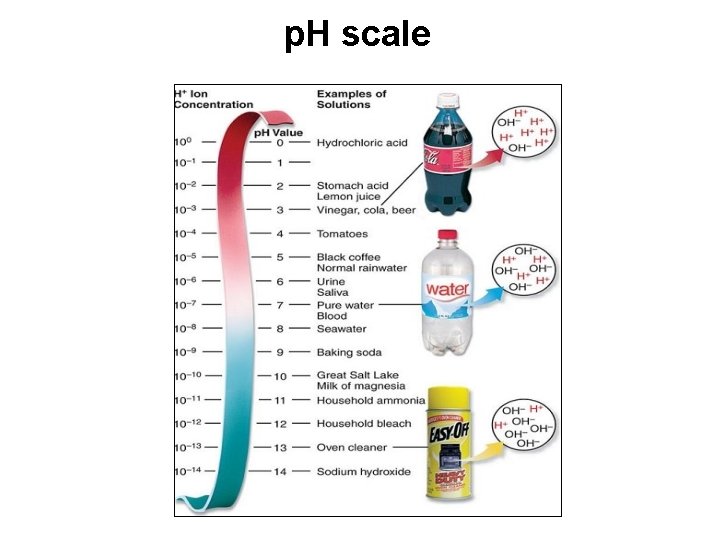
p. H scale
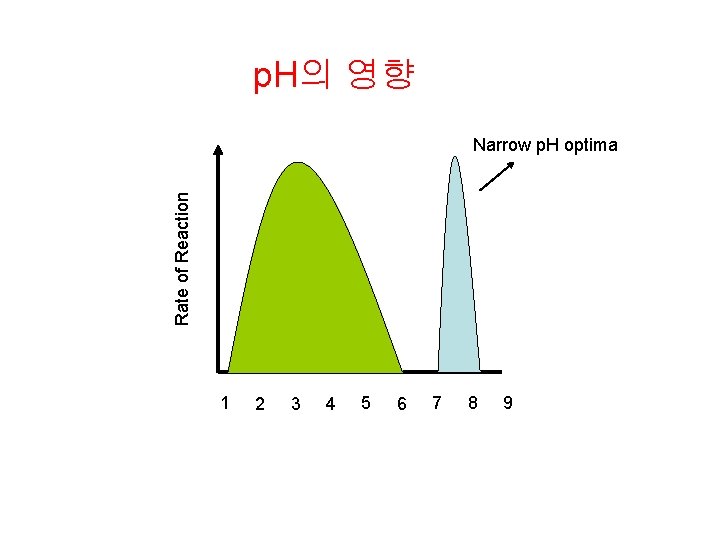
p. H의 영향 Rate of Reaction Narrow p. H optima 1 2 3 4 5 6 7 8 9
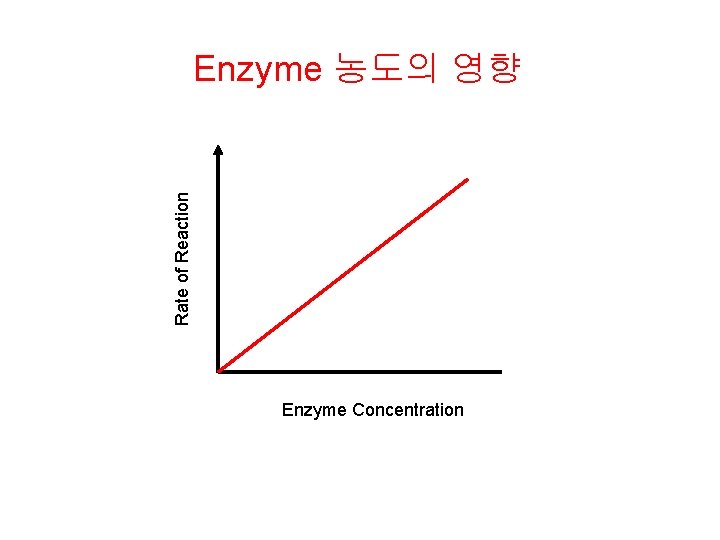
Rate of Reaction Enzyme 농도의 영향 Enzyme Concentration
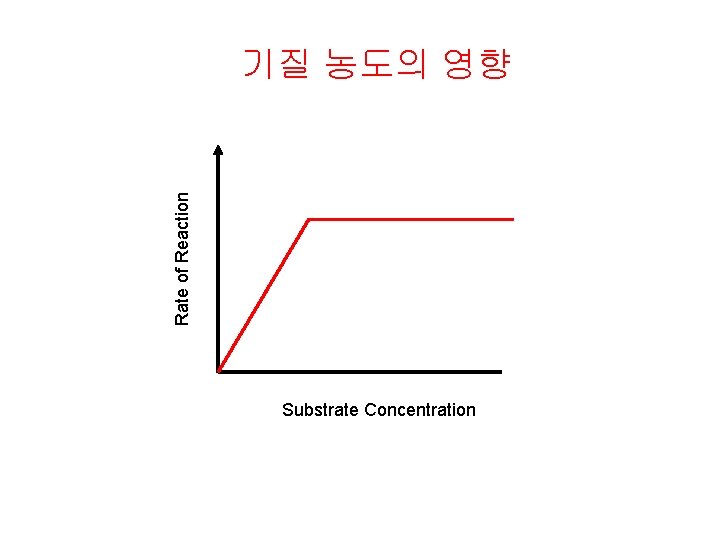
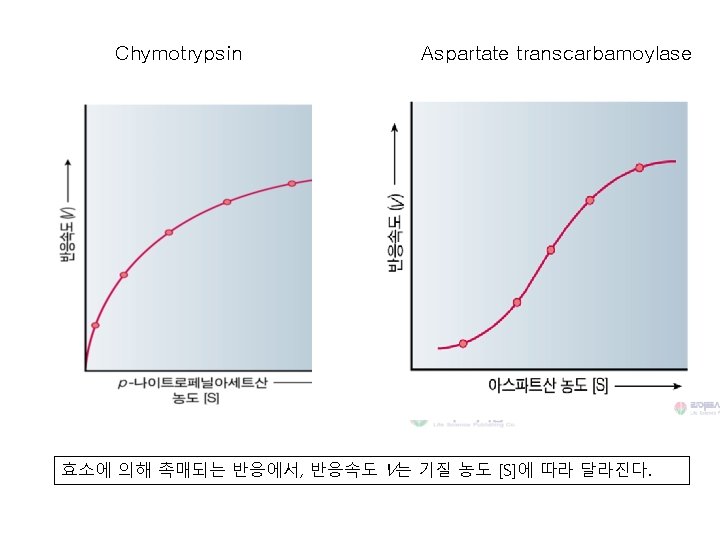
Rate of Reaction 기질 농도의 영향 Substrate Concentration

효소의 실생활에서의 활용 • Washing powders - lipase: greasy stains - protease: eggs, blood • Food industry - Fruit juices: using pectinase - 식혜: using amylase


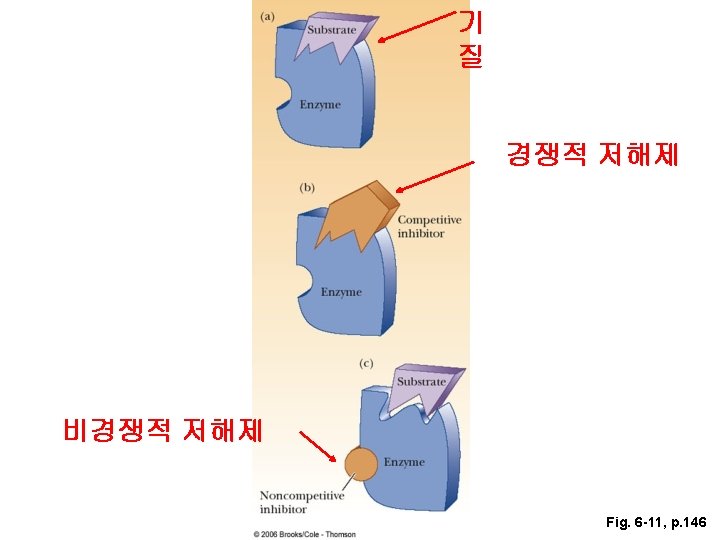
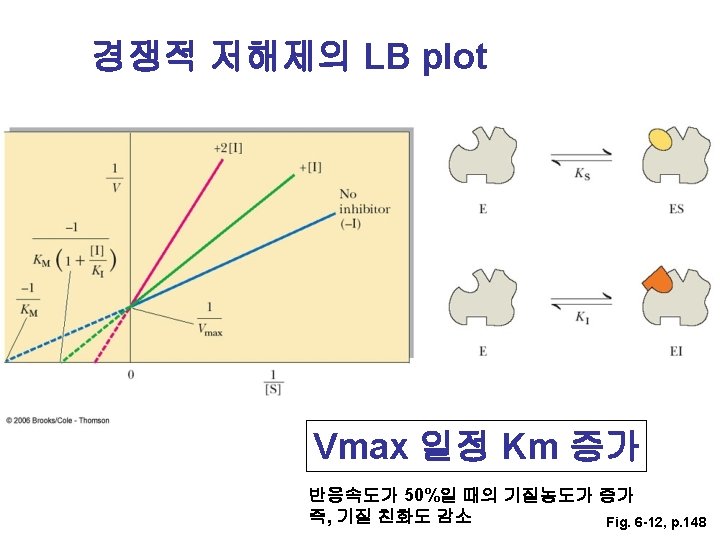

- Slides: 54