Enzyme Kinetics kcat Km Vmax S vo Km
![Enzyme Kinetics kcat / Km Vmax [S] vo= Km + [S] kcat Significance zero Enzyme Kinetics kcat / Km Vmax [S] vo= Km + [S] kcat Significance zero](https://slidetodoc.com/presentation_image_h2/74fbd296c91bbe734669a8963c492778/image-1.jpg)
![Significance of Enzyme Kinetics Obtain Vmax and Km 1 st order [S] = Low Significance of Enzyme Kinetics Obtain Vmax and Km 1 st order [S] = Low](https://slidetodoc.com/presentation_image_h2/74fbd296c91bbe734669a8963c492778/image-2.jpg)
![Km: Affinity with Substrate Km + [S] When using different substrate Vmax S 1 Km: Affinity with Substrate Km + [S] When using different substrate Vmax S 1](https://slidetodoc.com/presentation_image_h2/74fbd296c91bbe734669a8963c492778/image-3.jpg)
![t vo = [P] / min Unit = mmole/min Specific Activity Units Activity = t vo = [P] / min Unit = mmole/min Specific Activity Units Activity =](https://slidetodoc.com/presentation_image_h2/74fbd296c91bbe734669a8963c492778/image-8.jpg)
- Slides: 8
![Enzyme Kinetics kcat Km Vmax S vo Km S kcat Significance zero Enzyme Kinetics kcat / Km Vmax [S] vo= Km + [S] kcat Significance zero](https://slidetodoc.com/presentation_image_h2/74fbd296c91bbe734669a8963c492778/image-1.jpg)
Enzyme Kinetics kcat / Km Vmax [S] vo= Km + [S] kcat Significance zero order 1 st order Observe vo change under various [S], resulted plots yield Vmax and Km Turn over number Vmax k 3 [Et] 1 mmole min Specific Activity & E 3 E 2 E 1 Km Affinity with substrate Competitive Double reciprocal Bi-substrate reaction also follows M-M equation, but one of the substrate should be saturated when estimate the other Inhibition Maximum velocity Activity Unit unit mg Direct plot Non-competitive Uncompetitive Juang RH (2004) BCbasics
![Significance of Enzyme Kinetics Obtain Vmax and Km 1 st order S Low Significance of Enzyme Kinetics Obtain Vmax and Km 1 st order [S] = Low](https://slidetodoc.com/presentation_image_h2/74fbd296c91bbe734669a8963c492778/image-2.jpg)
Significance of Enzyme Kinetics Obtain Vmax and Km 1 st order [S] = Low → High vo = Vmax [S] Km + [S] E 3 E 2 E 1 Proportional to enzyme concentration zero order v 0 = Vmax × K = k 3 [Et] × K [S] = Fixed concentration Juang RH (2004) BCbasics
![Km Affinity with Substrate Km S When using different substrate Vmax S 1 Km: Affinity with Substrate Km + [S] When using different substrate Vmax S 1](https://slidetodoc.com/presentation_image_h2/74fbd296c91bbe734669a8963c492778/image-3.jpg)
Km: Affinity with Substrate Km + [S] When using different substrate Vmax S 1 S 2 S 3 1/2 If vo = Vmax 2 Vmax [S] = 2 Km + [S] = 2 [S] S 1 S 2 S 3 Km Affinity changes Km = [S] Juang RH (2004) BCbasics vo = Vmax [S]

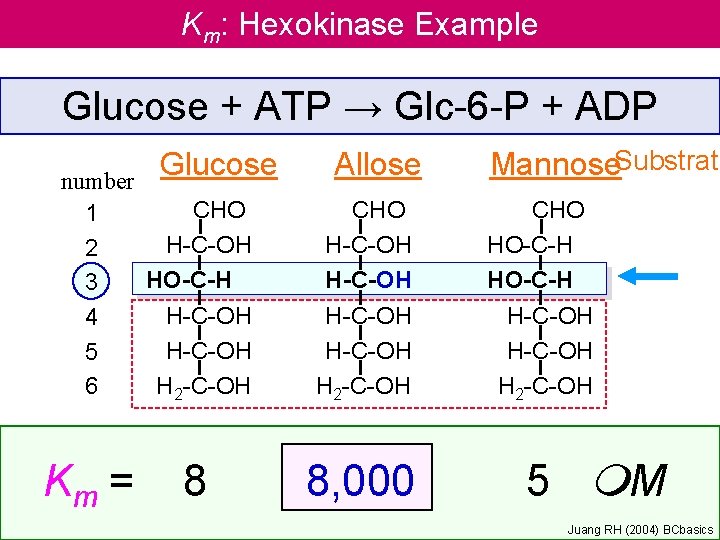
Km: Hexokinase Example Glucose + ATP → Glc-6 -P + ADP Glucose number CHO 1 H-C-OH 2 HO-C-H 3 H-C-OH 4 H-C-OH 5 6 H 2 -C-OH Km = 8 Allose CHO H-C-OH H 2 -C-OH 8, 000 Mannose. Substrate CHO HO-C-H H-C-OH H 2 -C-OH 5 m. M Juang RH (2004) BCbasics

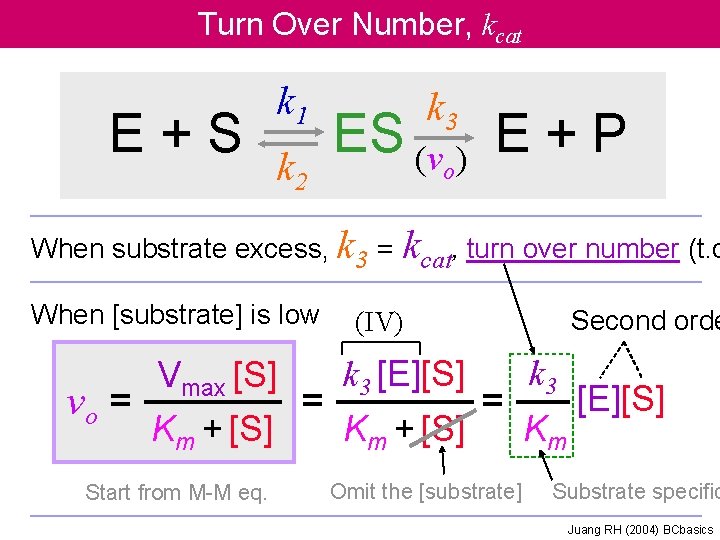
Turn Over Number, kcat E+S k 1 k 2 k 3 ES (v ) E + P o When substrate excess, k 3 = kcat, turn over number (t. o When [substrate] is low (IV) Second orde k 3 [E][S] Vmax [S] [E][S] vo = = = Km + [S] Km Start from M-M eq. Omit the [substrate] Substrate specific Juang RH (2004) BCbasics

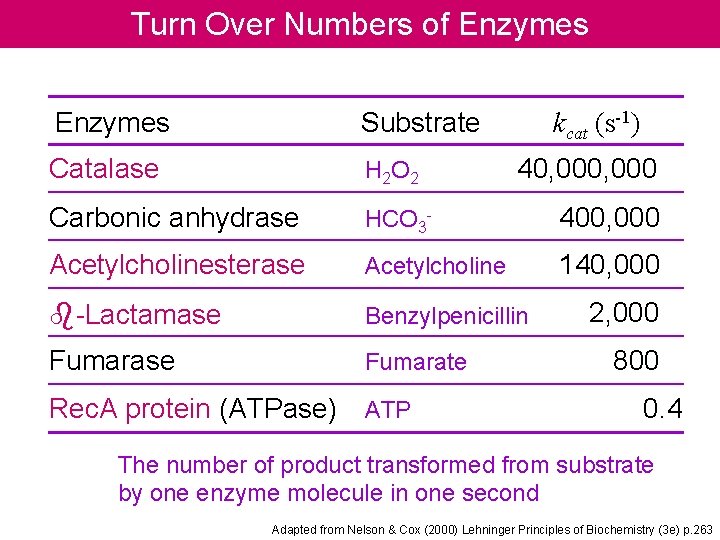
Turn Over Numbers of Enzymes Substrate Catalase H 2 O 2 Carbonic anhydrase HCO 3 - 400, 000 Acetylcholinesterase Acetylcholine 140, 000 b-Lactamase Benzylpenicillin Fumarase Fumarate Rec. A protein (ATPase) ATP kcat (s-1) 40, 000 2, 000 800 0. 4 The number of product transformed from substrate by one enzyme molecule in one second Adapted from Nelson & Cox (2000) Lehninger Principles of Biochemistry (3 e) p. 263

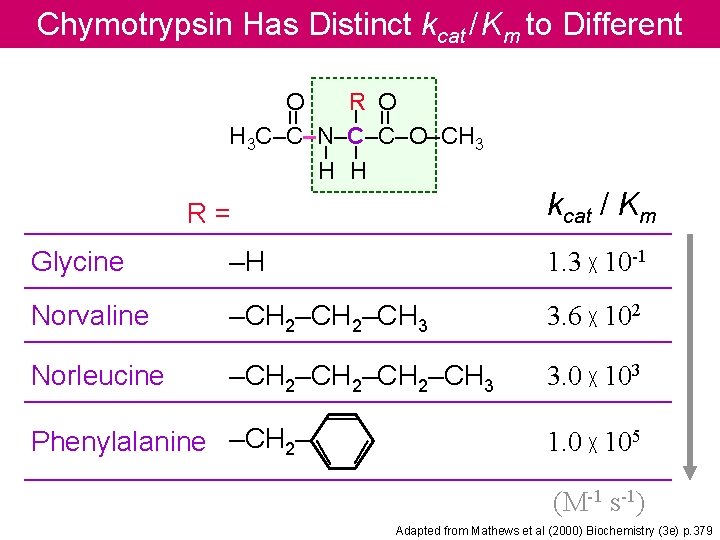
Chymotrypsin Has Distinct kcat / Km to Different Substrates = – – – = O R O H 3 C–C–N–C–C–O–CH 3 H H kcat / Km R= Glycine –H 1. 3 ╳ 10 -1 Norvaline –CH 2–CH 3 3. 6 ╳ 102 Norleucine –CH 2–CH 3 3. 0 ╳ 103 Phenylalanine –CH 2– 1. 0 ╳ 105 (M-1 s-1) Adapted from Mathews et al (2000) Biochemistry (3 e) p. 379
![t vo P min Unit mmolemin Specific Activity Units Activity t vo = [P] / min Unit = mmole/min Specific Activity Units Activity =](https://slidetodoc.com/presentation_image_h2/74fbd296c91bbe734669a8963c492778/image-8.jpg)
t vo = [P] / min Unit = mmole/min Specific Activity Units Activity = Protein (mg) Slope tan 0 10 20 30 40 Reaction time (min) y x y = tan x Juang RH (2004) BCbasics S → P mmole Product [P] Enzyme Activity Unit