Enzyme Inhibitors prevent enzymes from working There are
Enzyme Inhibitors prevent enzymes from working There are two types of inhibitor competitive non-competitive.

Inhibition Enzyme activity is influenced by the presence of other chemicals Enhance activity Cofactors: non-protein Coenzymes: organic molecules Deactivate activity Some molecules can work to stop an enzyme from working properly, inhibitors

Competitive Inhibitors Have a similar shape to the normal substrate and are able to bind to the active site. Do not react with the active site but leave after a time without any product forming. The rate of reaction decreases because the substrate molecules have to compete with the inhibitor for the active site. It is possible to reduce the effect of the inhibitor by adding more substrate
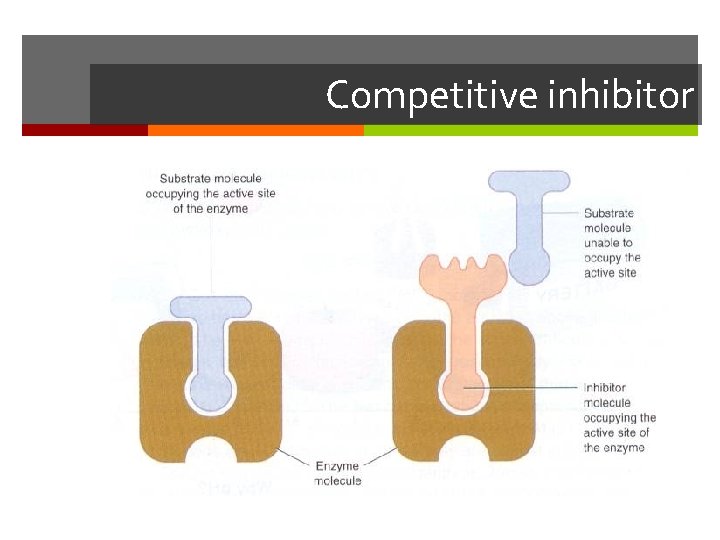
Competitive inhibitor
Competitive Inhibition This is when the inhibitor molecule competes directly with the substrate to occupy the active site. The bonding is not permanent, so eventually all substrate molecules will occupy active sites and be broken down, but it will take longer for the reaction to happen. Substrate Inhibitor Enzyme
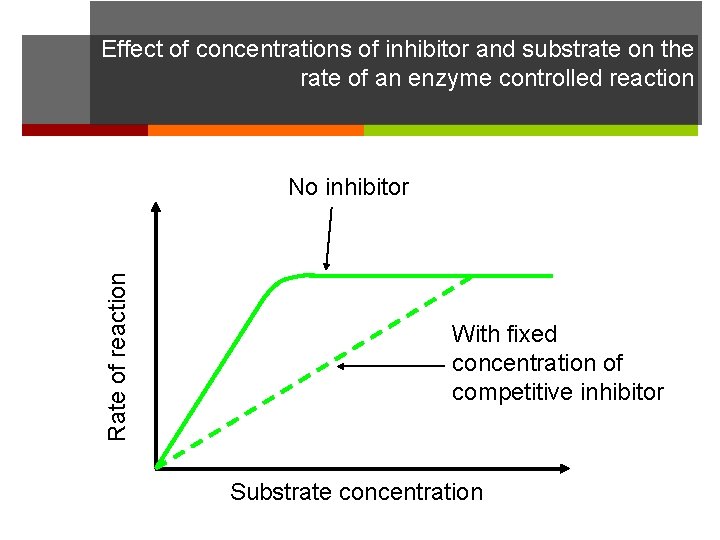
Effect of concentrations of inhibitor and substrate on the rate of an enzyme controlled reaction Rate of reaction No inhibitor With fixed concentration of competitive inhibitor Substrate concentration

Examples Competitive inhibitor Reversible Statins compete with a liver enzyme which helps to make cholesterol Non-reversible Penicillin inhibits an enzyme that makes cell walls in some bacteria
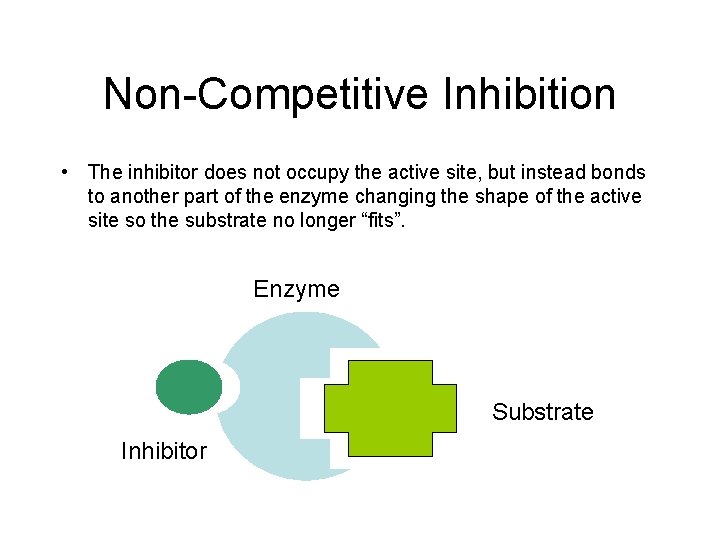
Non-Competitive Inhibition • The inhibitor does not occupy the active site, but instead bonds to another part of the enzyme changing the shape of the active site so the substrate no longer “fits”. Enzyme Substrate Inhibitor
Non-competitive inhibitors Molecules bind to some part of an enzyme other than the active site. This changes the active site so that the substrate can no longer fit. If the concentration of this type of inhibitor is high enough, all enzymes maybe inhibited and the reaction slows to nothing. Increasing the concentration of the substrate has no effect on this type of inhibition.
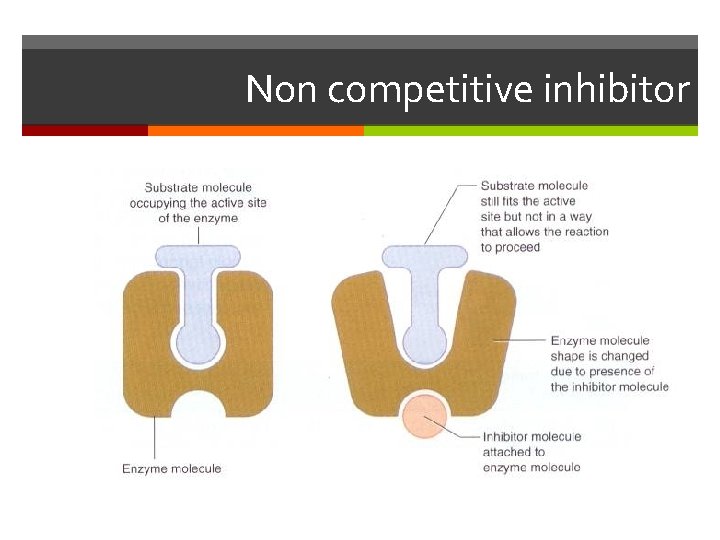
Non competitive inhibitor
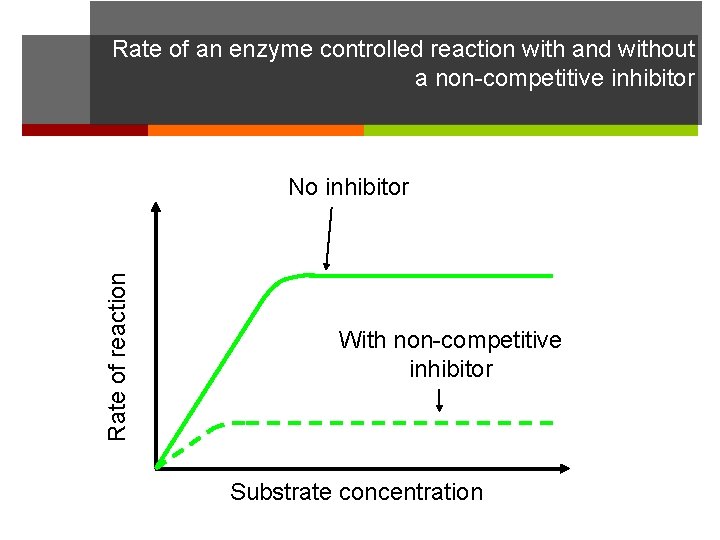
Rate of an enzyme controlled reaction with and without a non-competitive inhibitor Rate of reaction No inhibitor With non-competitive inhibitor Substrate concentration

Examples Non-competitive inhibitor Potassium cyanide bind to haem, which is part of cytochrome oxidase This is non-reversible
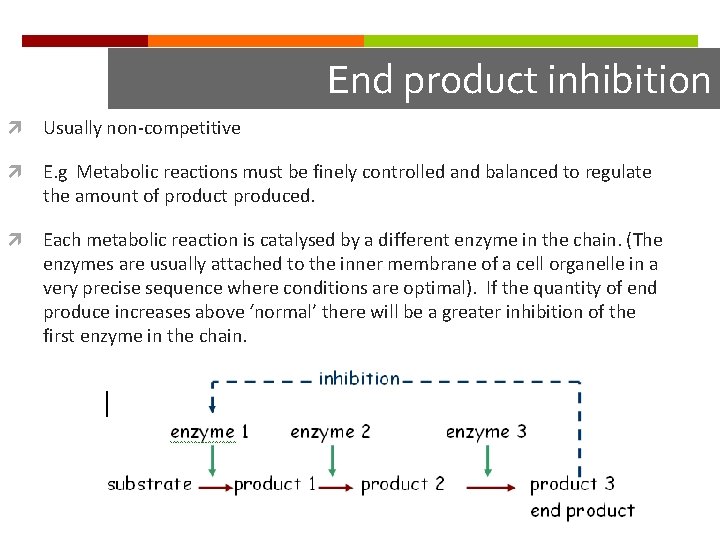
End product inhibition Usually non-competitive E. g Metabolic reactions must be finely controlled and balanced to regulate the amount of product produced. Each metabolic reaction is catalysed by a different enzyme in the chain. (The enzymes are usually attached to the inner membrane of a cell organelle in a very precise sequence where conditions are optimal). If the quantity of end produce increases above ‘normal’ there will be a greater inhibition of the first enzyme in the chain.

End product inhibition

Application of Inhibition • In real life inhibition can be used to regulate a metabolic pathway. If the end product of a chain of reactions inhibits the first enzyme in that pathway then the rate of reaction will slow down. • The inhibition is usually non-competitive and known as end-product inhibition.
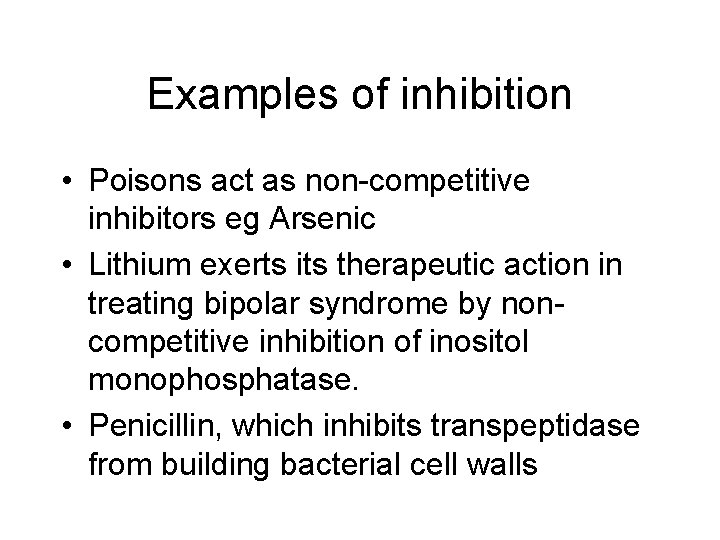
Examples of inhibition • Poisons act as non-competitive inhibitors eg Arsenic • Lithium exerts its therapeutic action in treating bipolar syndrome by noncompetitive inhibition of inositol monophosphatase. • Penicillin, which inhibits transpeptidase from building bacterial cell walls

End product inhibition • This is an example of non-competitive inhibition – product 3 binds to another part of the enzyme other than the active site. • It is also an example of a feedback mechanism.

Learning Outcomes • explain the importance of cofactors and coenzymes in enzyme-controlled reactions; • state that metabolic poisons may be enzyme inhibitors, and describe the action of one named poison; • state that some medicinal drugs work by inhibiting the activity of enzyme
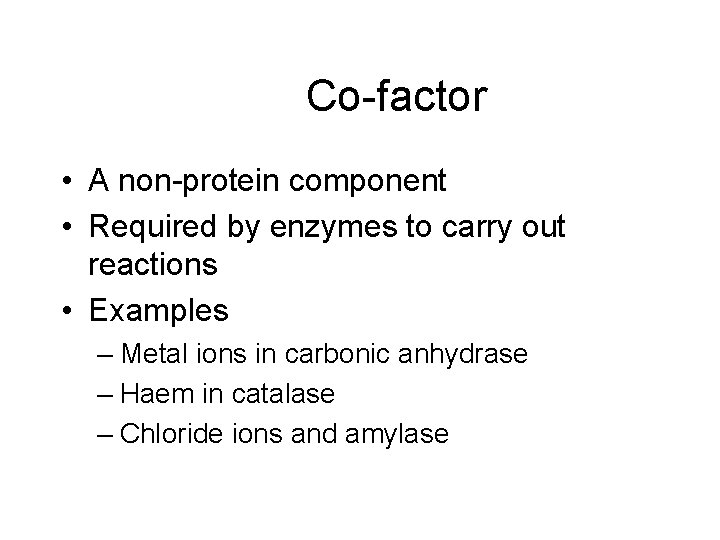
Co-factor • A non-protein component • Required by enzymes to carry out reactions • Examples – Metal ions in carbonic anhydrase – Haem in catalase – Chloride ions and amylase
Co-enzyme • Organic, non protein molecules • Role is to carry chemical groups between enzymes, linking together enzyme controlled reactions • Examples – NAD, FAD and coenzyme A – involved in respiration – NADP – involved in photosythesis

Prosthetic groups • A coenzyme that is a permanent part of the enzyme • Example – Carbonic anhydrase contains a zinc-based prosthetic group

Metabolic poisons • Metabolic poisons can be enzyme inhibitors • Example – Potassium cyanide • inhibits cell respiration • Non-competitive inhibitor for the enzyme cytochrome oxidase • Decreases the use of oxygen so that ATP can not be made • The organism respires anaerobically and lactic acid builds up in the blood
Medicines and enzymes • Infection by viruses are treated by using chemicals that act as protease inhibitors which the virus needs to build new viral coats. • Antibiotics – Penicillin inhibits a bacterial enzyme which makes bacterial cell walls
- Slides: 24