Enzymatic and Colorimetric Analyses 1 Analytical methods to


























- Slides: 57

Enzymatic and Colorimetric Analyses 1

Analytical methods to determine important parameters in the following sectors: Food & Beverage Environmental Clinical Pharmaceutical

Now internationally recognised as official methods because: They are simple to use They are fast (especially with automatic machines) They have low analysis costs The kits are long-life (if liquid)

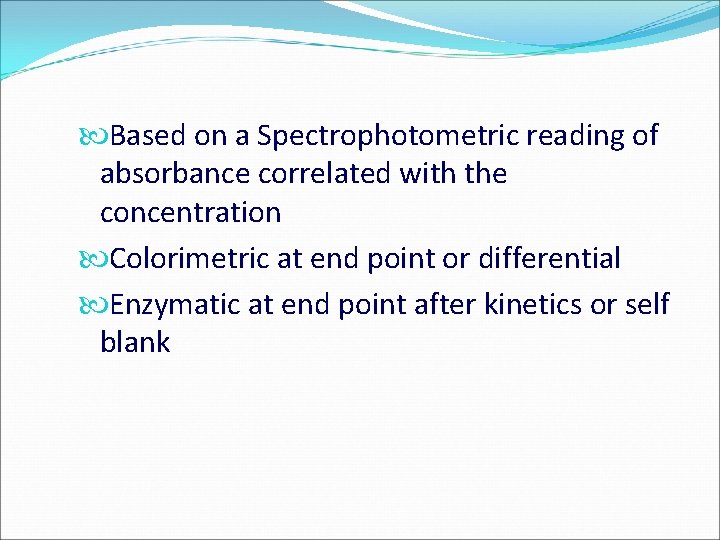
Based on a Spectrophotometric reading of absorbance correlated with the concentration Colorimetric at end point or differential Enzymatic at end point after kinetics or self blank

The principle parameters analysed are of particular importance as regards: Product stability Product genuineness Product transformation Product toxicity

Steroglass kit list • • • • Acetic acid D-Gluconic acid D-Lactic and L-Lactic acid D-Malic and L- Malic acid Pyruvic acid Tartaric acid Acetaldehyde CO 2 Anthocyanin Alpha Amino Nitrogen Ammoniacal Nitrogen Calcium Catechins • • • • Chlorides Colour Glycerol Glucose-Fructose Sucrose Iron Magnesium Polyphenols Potassium Copper Free SO 2 Total SO 2 L-Ascorbic

Kit List – the most popular and why. . ACETIC ACID: This is linked to product perishability and must be analysed before bottlingpackaging (alcoholic drinks).

Kit List – the most popular and why. . MALIC ACID: It is one of the organic acids responsible for secondary fermentation (e. g. malolactic fermentation in wine). Found naturally in some samples (e. g. apple juice) It must be analysed in the original product before bottling.

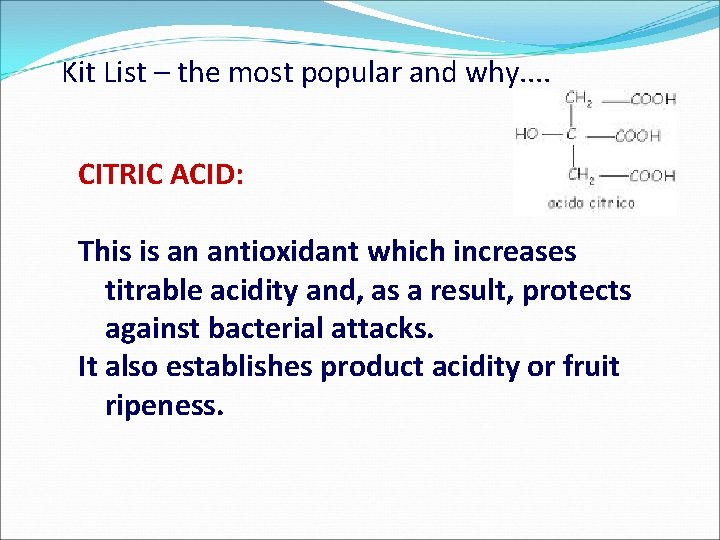
Kit List – the most popular and why. . CITRIC ACID: This is an antioxidant which increases titrable acidity and, as a result, protects against bacterial attacks. It also establishes product acidity or fruit ripeness.

Kit List – the most popular and why. . GLUCONIC ACID: This indicator develops when moulds are present in the product (and therefore a high possibility of degradation). It indicates a raw material or even the finished product is “rotten”.

Kit List – the most popular and why. . READILY ASSIMILABLE NITROGEN (R. A. N. ): SUM OF AMMONIACAL NITROGEN AND ALPHA AMINO NITROGEN It is fundamental because it “feeds” the yeasts and can ferment. . To avoid counterfeits (Amino/Ammoniacal ratio)

Kit List – the most popular and why. . R. A. N. : It replaces the FORMALIN n°!!!! Method used until now using FORMALIN which proved to be carcinogenic!

Kit List – the most popular and why. . It replaces the FORMALIN n°!!!! The Formalin no indicates when Nitrogen has combined with the aldehyde group of formaldehyde. It does not specify whether this is ammoniacal or alpha amino nitrogen. The yeasts prefer to eat the more readily assimilable ammoniacal nitrogen.

Kit List – the most popular and why. . It replaces the FORMALIN n°!!!! The Formalin no is analysed for Titration using concentrated Formaldehyde raised to p. H 8. 1 and titrated with Soda!

Kit List – the most popular and why. . SUGARS: GLUCOSE-FRUCTOSE-SUCROSE These are linked to the alcoholic strength of a drink (if it ferments) or to the sweetness and energy power of a product.

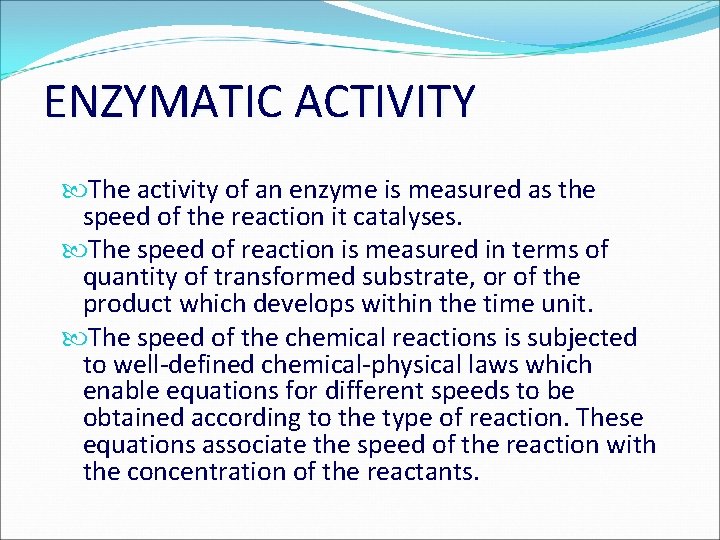
ENZYMATIC ACTIVITY The activity of an enzyme is measured as the speed of the reaction it catalyses. The speed of reaction is measured in terms of quantity of transformed substrate, or of the product which develops within the time unit. The speed of the chemical reactions is subjected to well-defined chemical-physical laws which enable equations for different speeds to be obtained according to the type of reaction. These equations associate the speed of the reaction with the concentration of the reactants.

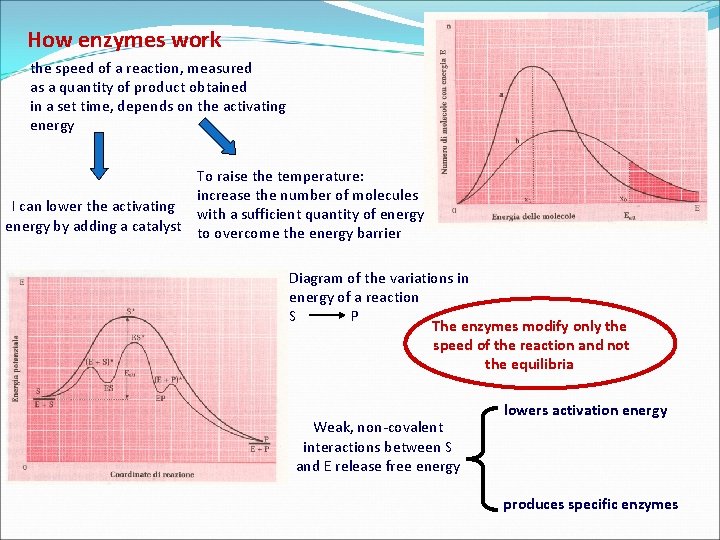
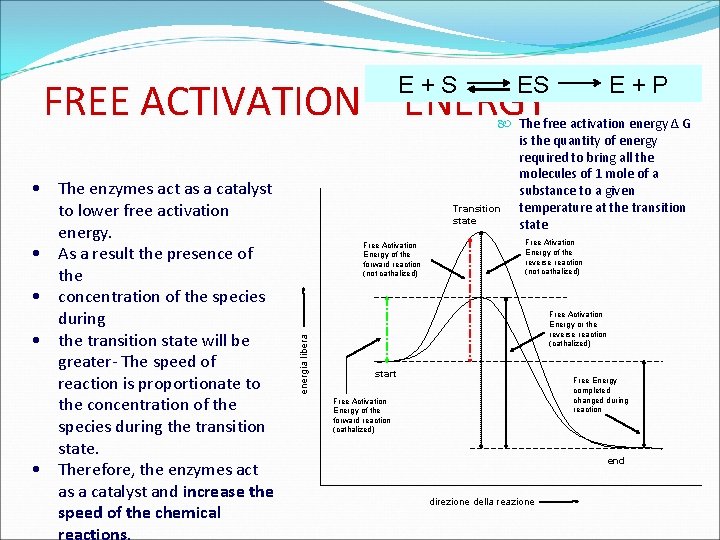
How enzymes work the speed of a reaction, measured as a quantity of product obtained in a set time, depends on the activating energy To raise the temperature: increase the number of molecules I can lower the activating with a sufficient quantity of energy by adding a catalyst to overcome the energy barrier Diagram of the variations in energy of a reaction S P The enzymes modify only the speed of the reaction and not the equilibria Weak, non-covalent interactions between S and E release free energy lowers activation energy produces specific enzymes

E+S ES E+P FREE ACTIVATION ENERGY Free Activation Energy of the forward reaction (not cathalized) energia libera • The enzymes act as a catalyst to lower free activation energy. • As a result the presence of the • concentration of the species during • the transition state will be greater- The speed of reaction is proportionate to the concentration of the species during the transition state. • Therefore, the enzymes act as a catalyst and increase the speed of the chemical The free activation energy Δ G is the quantity of energy required to bring all the molecules of 1 mole of a substance to a given Transition temperature at the transition state Free Ativation Energy of the reverse reaction (not cathalized) Free Activation Energy or the reverse reaction (cathalized) start Free Energy completed changed during reaction Free Activation Energy of the forward reaction (cathalized) end direzione della reazione

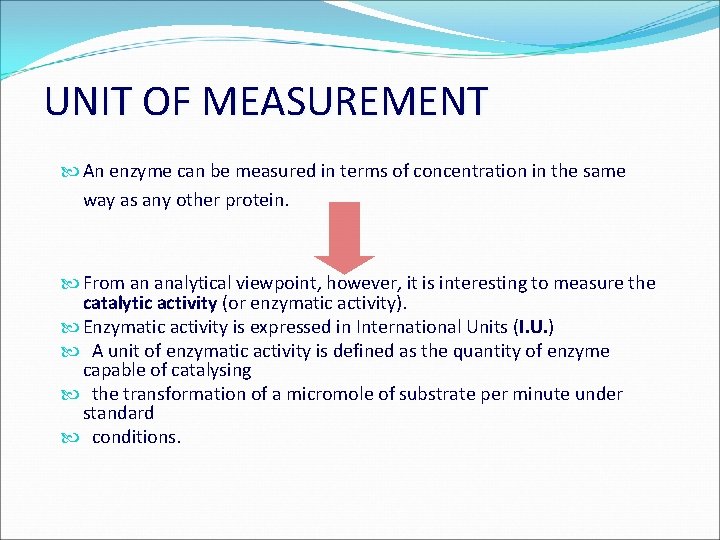
UNIT OF MEASUREMENT An enzyme can be measured in terms of concentration in the same way as any other protein. From an analytical viewpoint, however, it is interesting to measure the catalytic activity (or enzymatic activity). Enzymatic activity is expressed in International Units (I. U. ) A unit of enzymatic activity is defined as the quantity of enzyme capable of catalysing the transformation of a micromole of substrate per minute under standard conditions.

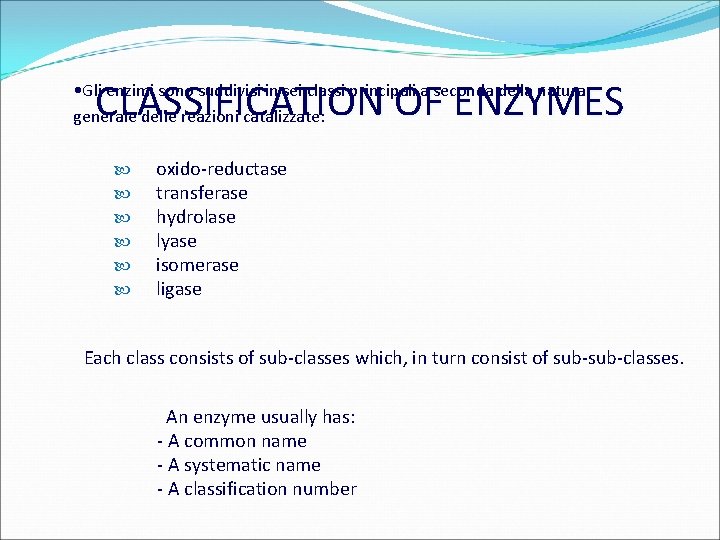
CLASSIFICATION OF ENZYMES • Gli enzimi sono suddivisi in sei classi principali a seconda della natura generale delle reazioni catalizzate: oxido-reductase transferase hydrolase lyase isomerase ligase Each class consists of sub-classes which, in turn consist of sub-classes. An enzyme usually has: - A common name - A systematic name - A classification number

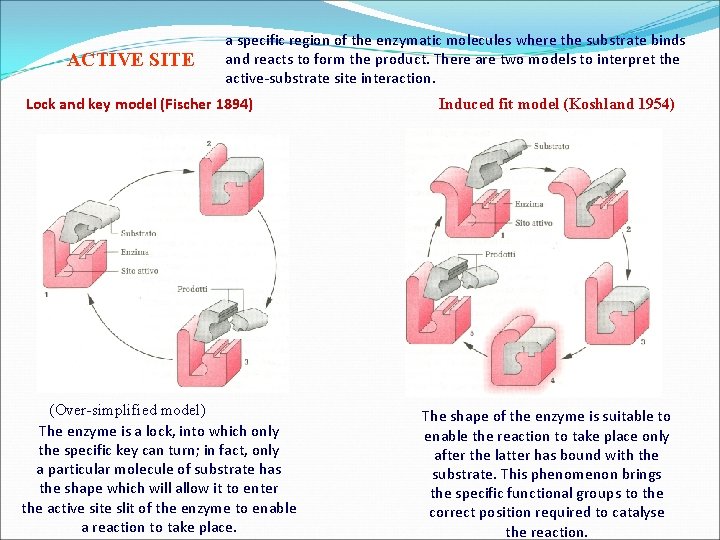
ACTIVE SITE a specific region of the enzymatic molecules where the substrate binds and reacts to form the product. There are two models to interpret the active-substrate site interaction. Lock and key model (Fischer 1894) (Over-simplified model) The enzyme is a lock, into which only the specific key can turn; in fact, only a particular molecule of substrate has the shape which will allow it to enter the active site slit of the enzyme to enable a reaction to take place. Induced fit model (Koshland 1954) The shape of the enzyme is suitable to enable the reaction to take place only after the latter has bound with the substrate. This phenomenon brings the specific functional groups to the correct position required to catalyse the reaction.

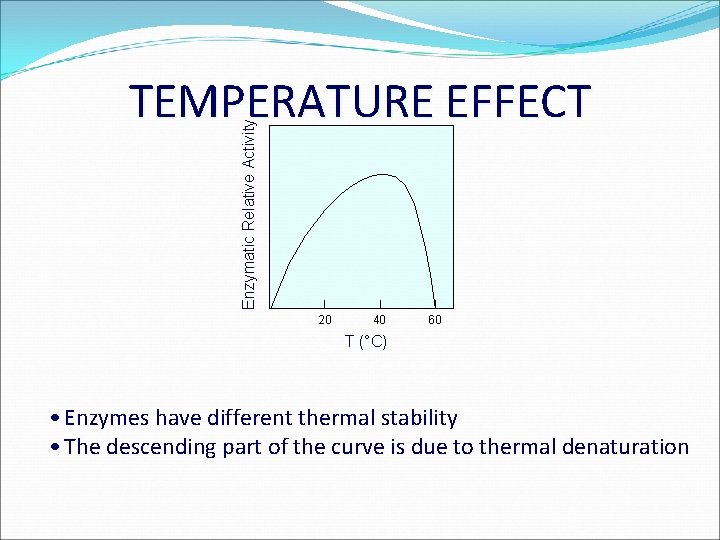
Enzymatic Relative Activity TEMPERATURE EFFECT 20 40 60 T (°C) • Enzymes have different thermal stability • The descending part of the curve is due to thermal denaturation

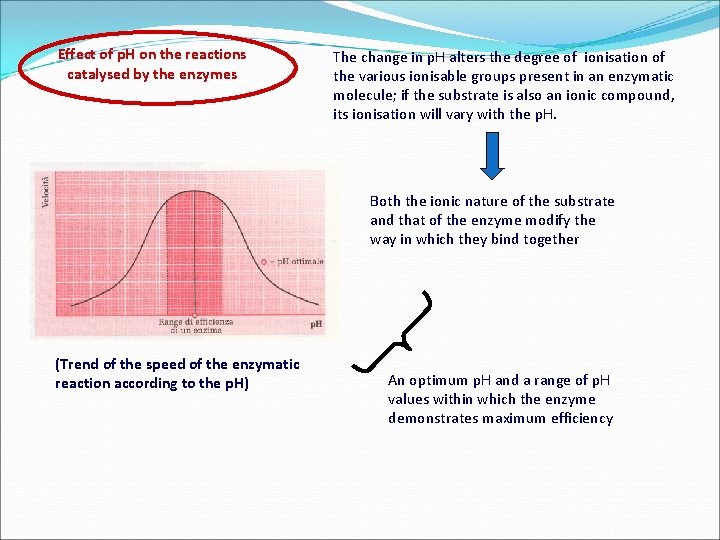
Effect of p. H on the reactions catalysed by the enzymes The change in p. H alters the degree of ionisation of the various ionisable groups present in an enzymatic molecule; if the substrate is also an ionic compound, its ionisation will vary with the p. H. Both the ionic nature of the substrate and that of the enzyme modify the way in which they bind together (Trend of the speed of the enzymatic reaction according to the p. H) An optimum p. H and a range of p. H values within which the enzyme demonstrates maximum efficiency

How to obtain kinetic data Spectrophotometric methods Fluorimetric methods Potenziometric methods (p. H) Manometric methods Radio-isotopic methods Continuous methods Discontinuous methods Joint methods

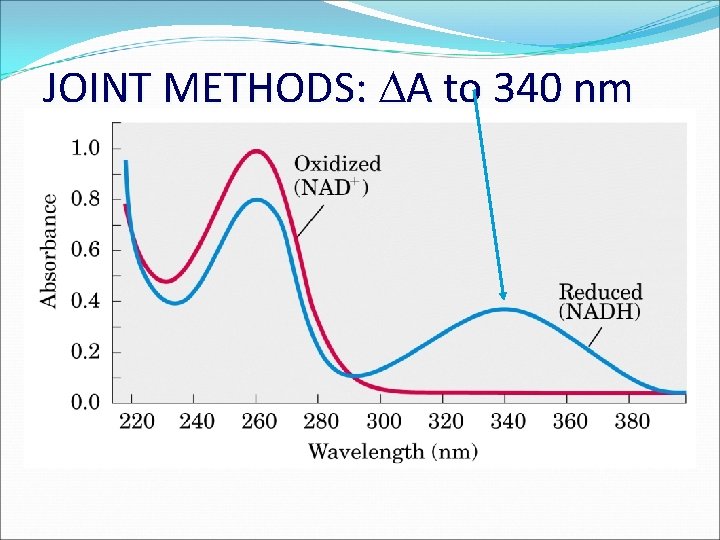
JOINT METHODS: A to 340 nm

WINE Processing and ANALYTICAL checks

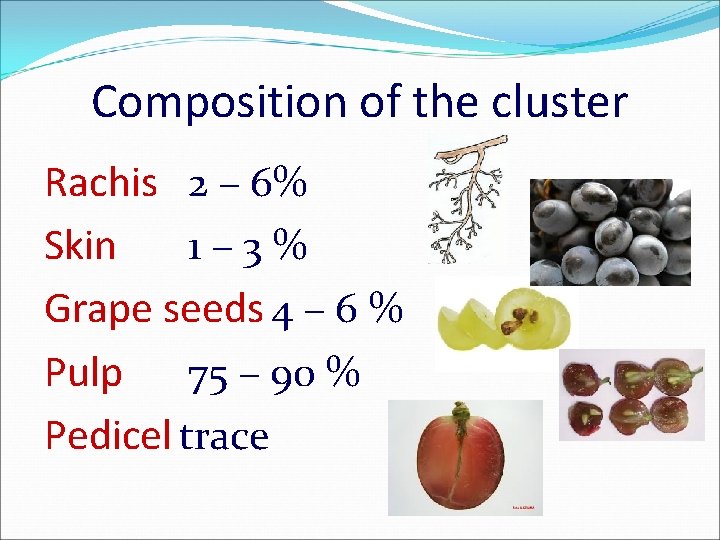
Composition of the cluster Rachis 2 – 6% Skin 1 – 3 % Grape seeds 4 – 6 % Pulp 75 – 90 % Pedicel trace

Contribution to the wine Rachis: Skin: Pulp: polyphenols (pigments), minerals polyphenols water 70 -90% sugars organic acids

What to check for grape ripeness Sugar content Titratable acidity Polyphenolic maturity

Vinification Stripping Pressing Must = check analysis fermentable sugars R. A. N. Titrable acidity Gluconic Acid Enzymatic treatment (? )

Wine is produced from sugar solutions obtained by crushing the bunch of grapes left to ferment with unicellular yeasts from the Saccharomyces genus present in the grape skin or from selected cultures. The characteristic organoleptic qualities (colour, flavour, bouquet) are differentiated by the conditions of fermentation. These become more pronounced during the subsequent stages in the process. Under anaerobic conditions, yeast transforms 100 grams of sugar into 51. 1 of spirit. This is the ideal yield. In reality, part of the sugar available is used by the yeast to multiply. Furthermore, the must yeasts produce not only alcohol and carbon dioxide during fermentation, but also secondary products (glycerol, acetic acid, succinic acid) which contribute towards giving the finished product its characteristic bouquet.

Fermentation Spontaneous autochthonous yeasts Induced with selected yeasts Competition between Yeasts – Bacteria

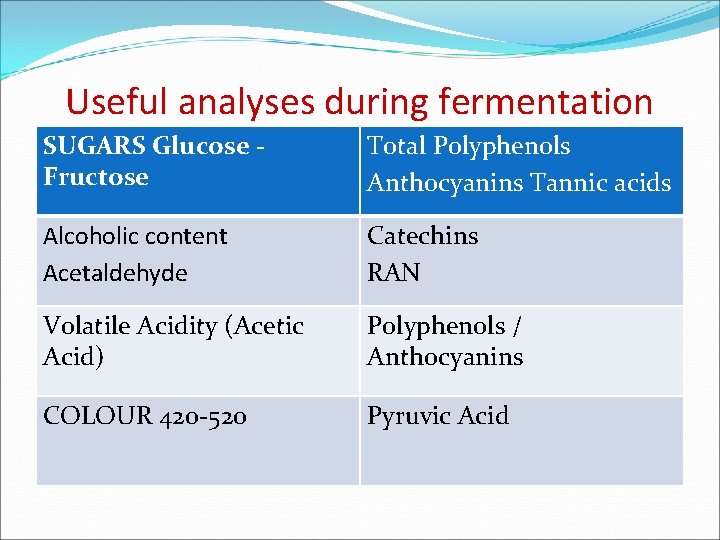
Useful analyses during fermentation SUGARS Glucose Fructose Total Polyphenols Anthocyanins Tannic acids Alcoholic content Acetaldehyde Catechins RAN Volatile Acidity (Acetic Acid) Polyphenols / Anthocyanins COLOUR 420 -520 Pyruvic Acid

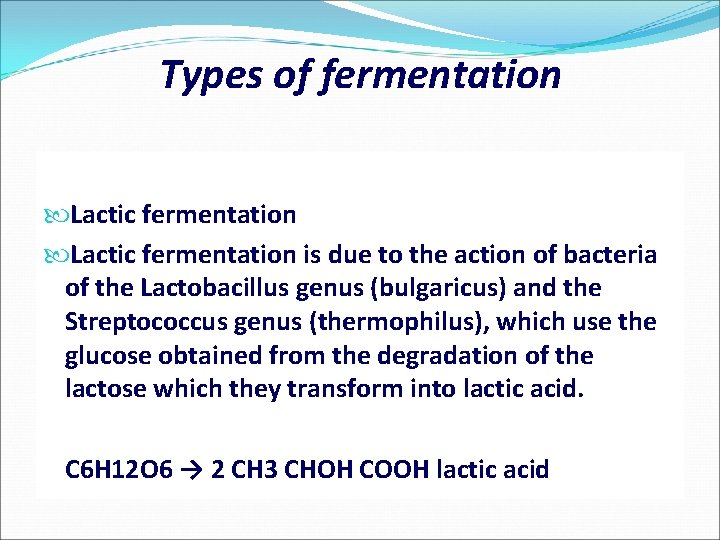
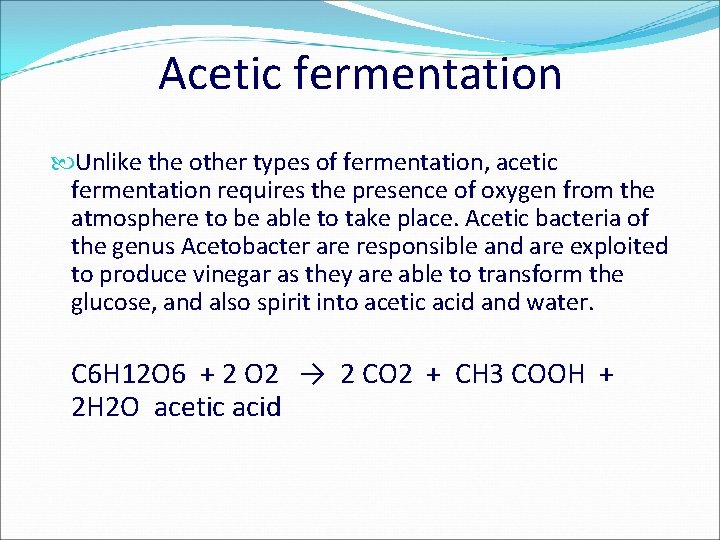
TYPES OF FERMENTATION Lactic Fermentation Alcoholic Fermentation Acetic Fermentation

FERMENTATION From a strictly chemical viewpoint, fermentation is an anaerobic oxidation process by numerous organisms depending on glucides to produce energy. As this type of metabolism is so important in the preparation of numerous foods, the term fermentation has been widely used to indicate any transformation catalysed by a micro-organism. Sometimes the term is still used in this sense. The word fermentation comes from the Latin fervere (simmer), a term used to describe the appearance of the must as the wine is prepared. Louis Pasteur gave a better explanation saying that fermentation was due to as yet not clearly identified bodies described as ferments, contained in yeasts. Subsequent studies discovered the protein content of the ferments and identified them as containing enzymes which enable the fermentative processes. Later, the main paths of energy metabolism in general and of fermentation in particular were clarified.

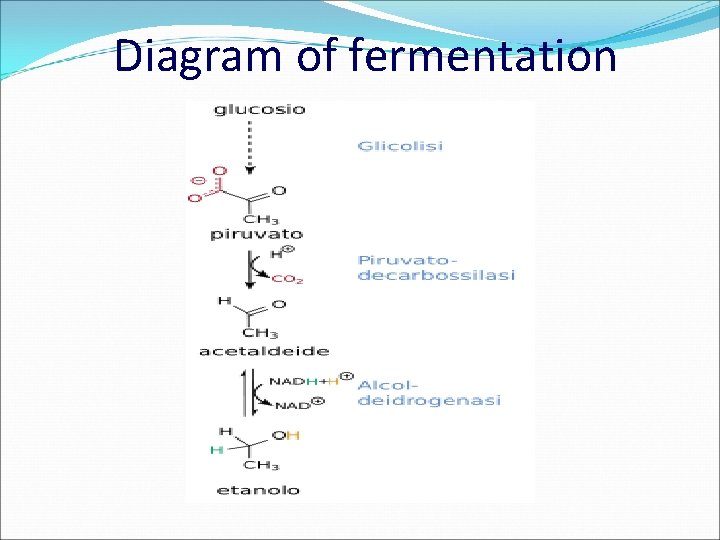
ALCOHOLIC FERMENTATION Alcoholic fermentation is a form of energy metabolism which occurs in some yeasts in the absence of oxygen. This type of fermentation is due to the action of yeasts belonging to the Saccharomyces genus, of which the most well-known is S. cerevisiae, found on the grape skin and in beer yeast, which transform glucose into spirit or ethanol and carbon dioxide.

Diagram of fermentation

The alcohol produced acts as an antiseptic against the other micro-organisms present, which die when the concentration of alcohol increases. C 6 H 12 O 6 — 2 CO 2 + CH 3 CH 2 OH ethilic alcohol Alcoholic fermentation is used to produce wine, beer, champagne, alcoholic drinks in general and in bread making.

Production of alcohol C 12 H 22 O 11+ H 2 O = C 6 H 12 O 6 + C 6 H 12 O 6 -> 2 CH 3 CH 2 OH + CO 2 + energia Other types of fermentation: Malolactic Pyruvic Acetic

Types of fermentation Lactic fermentation is due to the action of bacteria of the Lactobacillus genus (bulgaricus) and the Streptococcus genus (thermophilus), which use the glucose obtained from the degradation of the lactose which they transform into lactic acid. C 6 H 12 O 6 → 2 CH 3 CHOH COOH lactic acid

Acetic fermentation Unlike the other types of fermentation, acetic fermentation requires the presence of oxygen from the atmosphere to be able to take place. Acetic bacteria of the genus Acetobacter are responsible and are exploited to produce vinegar as they are able to transform the glucose, and also spirit into acetic acid and water. C 6 H 12 O 6 + 2 O 2 → 2 CO 2 + CH 3 COOH + 2 H 2 O acetic acid

Devatting - Filtration Addition of sulphur (gas or solid) Combination demand Acetaldehyde Total SO 2 Free SO 2 Combined SO 2

Physical treatments Acidification Disacidification Demetallisation ( Cu, Fe, Ca ) Stabilisation: Tartaric Protein

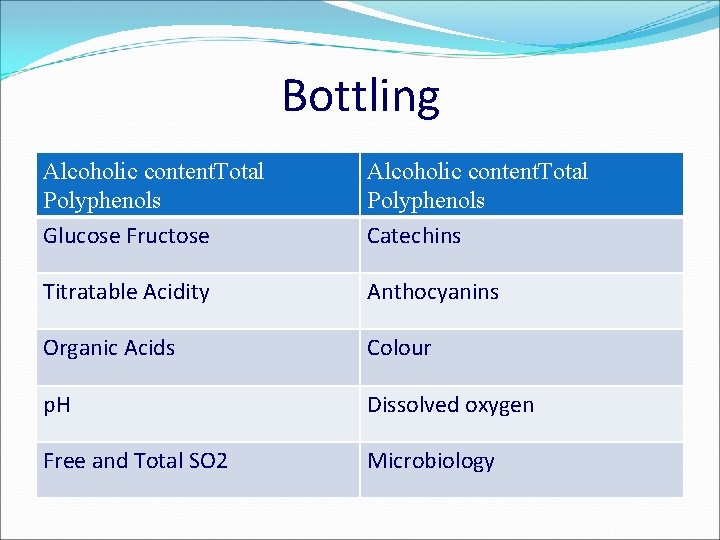
Bottling Alcoholic content. Total Polyphenols Glucose Fructose Alcoholic content. Total Polyphenols Catechins Titratable Acidity Anthocyanins Organic Acids Colour p. H Dissolved oxygen Free and Total SO 2 Microbiology

STEROGLASS KITS: Advantages Ready to use (Liquid) Easy to use Stable and reproducible (automatic) Separate Standards (and multiparametric) Long expiry dates (minimum 2 years) "Unbeatable" prices Analytical Application Support Continual Research and Development

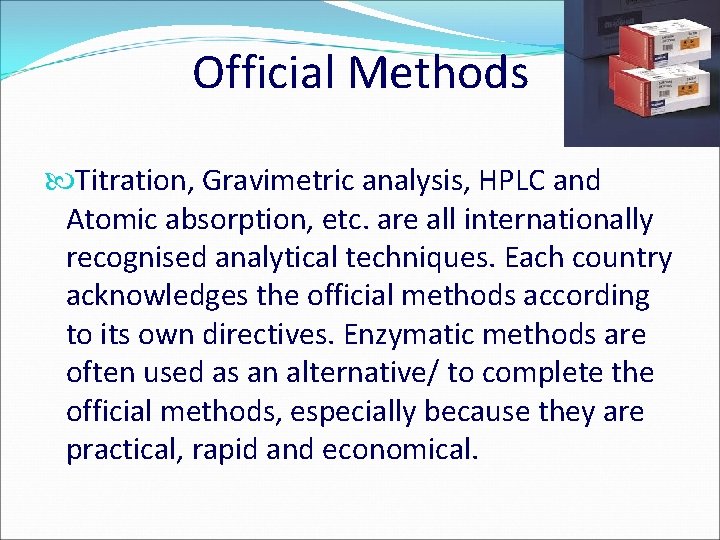
Official Methods Titration, Gravimetric analysis, HPLC and Atomic absorption, etc. are all internationally recognised analytical techniques. Each country acknowledges the official methods according to its own directives. Enzymatic methods are often used as an alternative/ to complete the official methods, especially because they are practical, rapid and economical.

Official Methods – e. g. Wine OIV and Official Gazette. The following enzymatic kits are officially recognised: Glucose and Fructose Lactic Acid Citric Acid L-Malic and D-Malic Acid

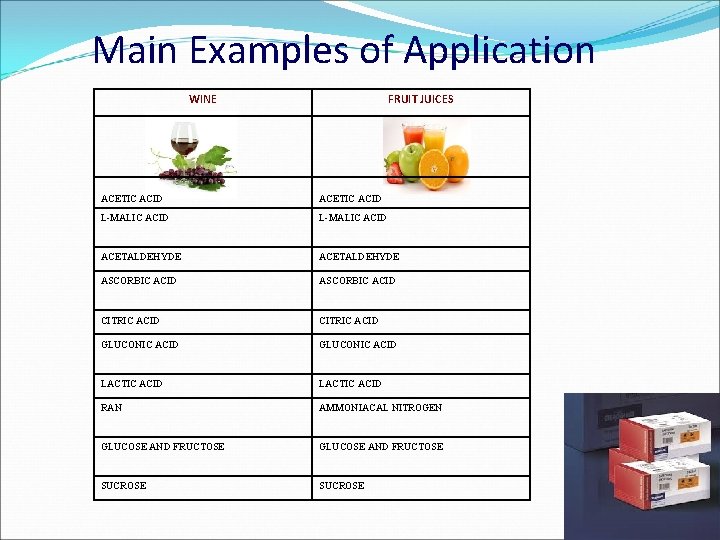
Main Examples of Application WINE FRUIT JUICES ACETIC ACID L-MALIC ACID ACETALDEHYDE ASCORBIC ACID CITRIC ACID GLUCONIC ACID LACTIC ACID RAN AMMONIACAL NITROGEN GLUCOSE AND FRUCTOSE SUCROSE

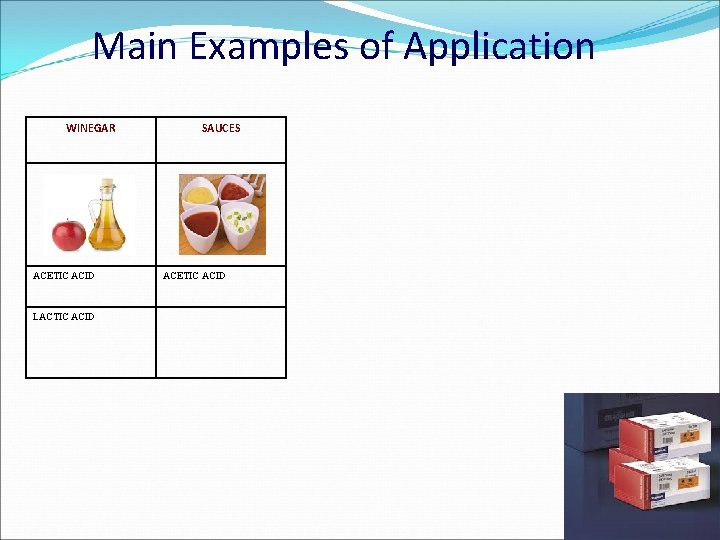
Main Examples of Application WINEGAR ACETIC ACID LACTIC ACID SAUCES ACETIC ACID

Main Examples of Application BEER CHEESES ACETIC ACID ASCORBIC ACID ACETALDEHYDE GLUCONIC ACID ASCORBIC ACID LACTIC ACID CITRIC ACID RAN GLUCOSE AND FRUCTOSE SUCROSE

Main Examples of Application YOGURT MAYONNAISE ACETIC ACID ACETALDEHYDE CITRIC ACID LACTIC ACID

Main Examples of Application FRUIT AND VEGETABLE PRODUCTS MALIC ACID HOMOGENISED BABY FOODS SUCROSE BREAD AND BAKERY PRODUCTS ACETALDEHYDE COFFEE ACETALDEHYDE

Main Examples of Application COCOA ACETALDEHYDE TOBACCO SUCROSE TEA ACETALDEHYDE POTATOES ASCORBIC ACID SUCROSE

Main Examples of Application FLOUR MEAT BY-PRODUCTS ASCORBIC ACID RAN GLUCONIC ACID LACTIC ACID RAN SUCROSE OIL, MARGARINE CITRIC ACID COSMETICS CITRIC ACID LACTIC ACID

Main Examples of Application DETERGENTS CITRIC ACID LIQUEURS GLUCOSE AND FRUCTOSE JAMS SUCROSE GLUCOSE AND FRUCTOSE HONEY GLUCOSE AND FRUCTOSE

Main competitors R-Biopharm (formerly Boheringer, formerly Difchamb) Megazyme

THANKS FOR YOUR ATTENTION 57