Environmental Systems Topic 1 Systems and Models Statements








- Slides: 19

Environmental Systems Topic - 1 Systems and Models Statements 1. A – 1. G

What is Environmental Systems About? • In this course we will be studying and analyzing the environment as a set of complex systems. • We won’t look at them separately as a biologist, geologist, or earth scientist would but as all three professions combined.

So what is a system? • Systems are sets of entities that function as a whole. • A group of parts connected together in an organized way to make a more complex entity.

Examples of Systems

The System that we will study most…. • The Ecosystem - a community of organisms in its abiotic environment, together with the relationships amongst these components.

Every system has a boundary Outside of that boundary lies the system’s environment. Environment: everything outside of a system that can influence the system or can be influenced by the system. -Therefore different systems can be linked.

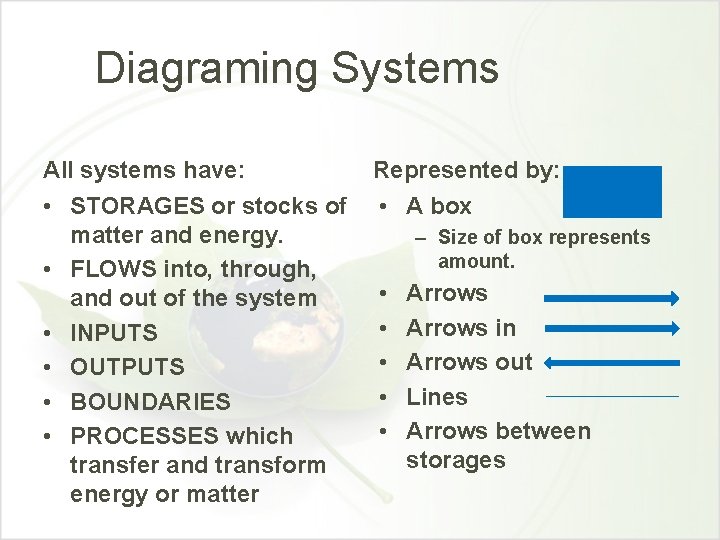
Diagraming Systems All systems have: • STORAGES or stocks of matter and energy. • FLOWS into, through, and out of the system • INPUTS • OUTPUTS • BOUNDARIES • PROCESSES which transfer and transform energy or matter Represented by: • A box – Size of box represents amount. • • • Arrows in Arrows out Lines Arrows between storages

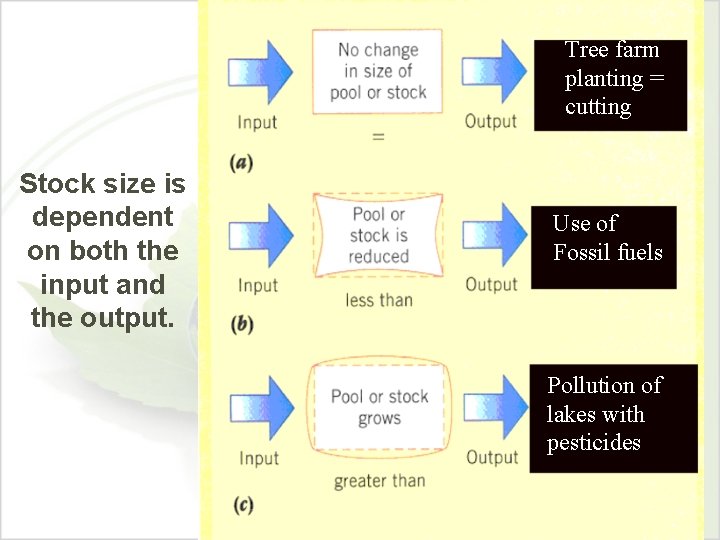
Tree farm planting = cutting Stock size is dependent on both the input and the output. Use of Fossil fuels Pollution of lakes with pesticides

Transfers within a system • Transfer when matter or energy is moved from one location to another. • Examples?

Transformations within a System • Transformation interaction within a system that forms a new end product or involves a change in state. • Examples?

Open Systems • A type of system that exchanges matter and energy across its boundary with its environment. • Therefore, these systems exchange factors with other systems around them. • Almost all systems are open systems.

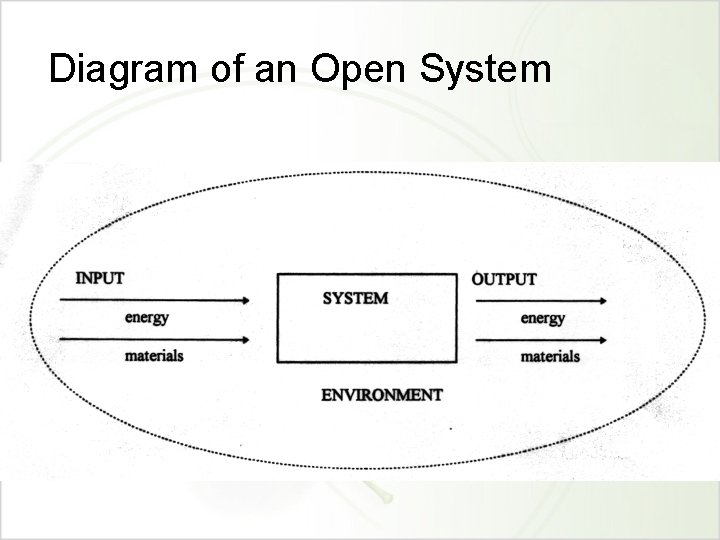
Diagram of an Open System

Closed System • A system that exchanges energy with its environment but not matter. • Closed systems are not found in nature. – But globally, the cycles of matter can be thought of as closed systems – Ex: • How could you diagram a closed system?

Isolated Systems • A System that exchanges neither matter nor energy with its environment. • This cannot exist. • Possible exception? ? ?

The First Law of Thermodynamics • First Law - Energy may not be created or destroyed, but may change from one form to another. – AKA - The Law of Conservation of Energy • What does this mean? • Examples?

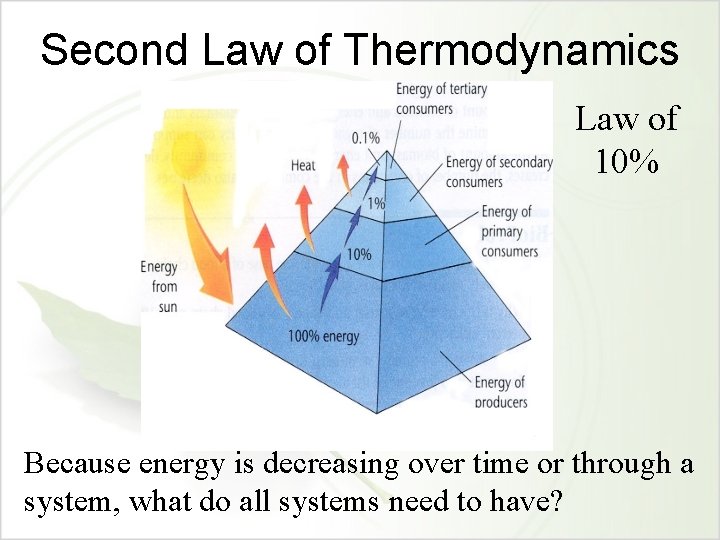
The Second Law of Thermodynamics • What is needed for any system to be sustaining? • What happens to energy in a system? • This is called the Second Law of Thermodynamics – Entropy or disorder in a system increases over time. – Why? • Eventually, all energy is emitted back into the environment as unusable heat, but it is NOT destroyed. – Example?

Second Law of Thermodynamics Law of 10% Because energy is decreasing over time or through a system, what do all systems need to have?

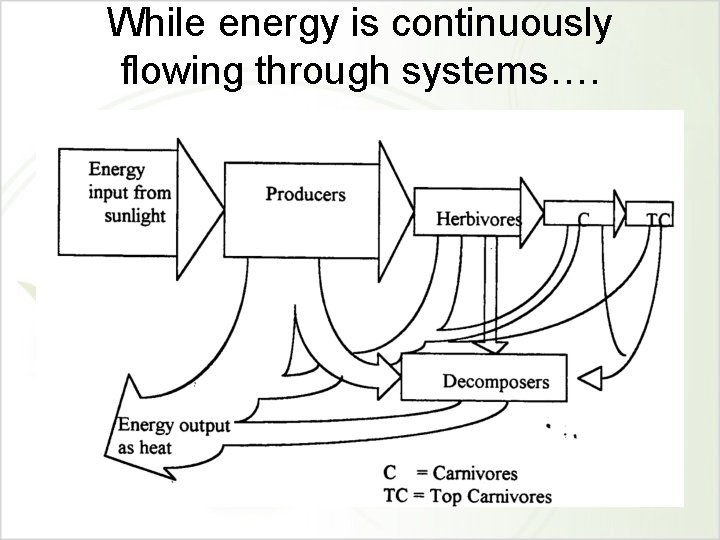
While energy is continuously flowing through systems….

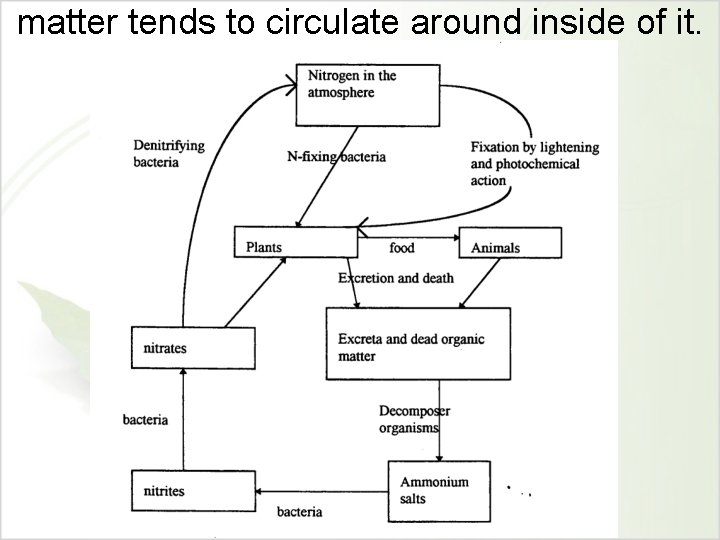
matter tends to circulate around inside of it.