Environmental Systems Topic 1 Systems and Models Assessment











- Slides: 19

Environmental Systems Topic - 1 Systems and Models Assessment Statements 1. 1. 1 -1. 1. 4

So What is Environmental Systems About? • In this course we will be studying and analyzing the environment as a set of complex systems. • We won’t look at them separately as a biologist, geologist, or earth scientist would - but as all three professions combined.

So what is a system? • Systems are sets of entities that function as a whole; they can be isolated physically or conceptually. • A group of parts connected together in an organized way to make a more complex entity.

Examples of Systems

The System that we will study most…. • The Ecosystem - a community of organisms in its abiotic environment, together with the relationships amongst these components.

Every system has a boundary Outside of that boundary lies the system’s environment. Environment: everything outside the system that can influence the system or in turn can be influenced by the system.

3 Types of Systems • Open • Closed • Isolated

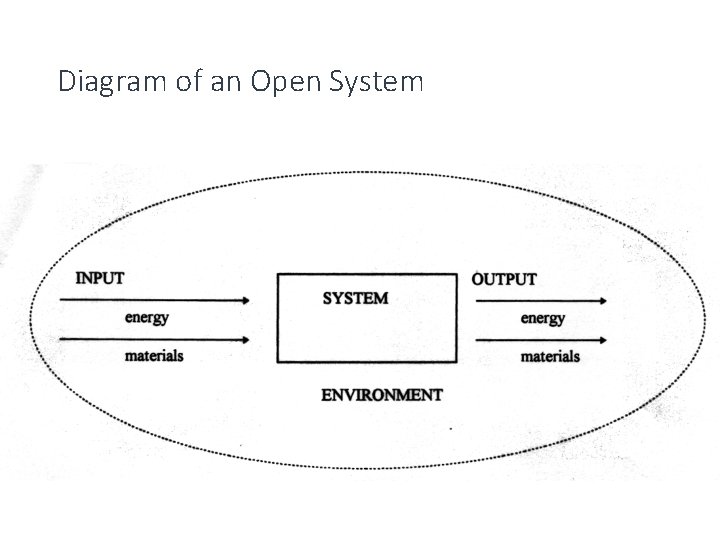
Open Systems • A type of system that exchanges matter and energy across its boundary with its environment. • Therefore, these systems exchange factors with other systems around them. • Almost all systems are open systems.

Diagram of an Open System

Closed System • A system that exchanges energy with its environment but not matter. • Closed systems are not found in nature. • (But globally, the cycles of matter can be thought of as closed systems) • Ie: • How could you diagram a closed system?

The Earth is a system, but is it an open or closed one?

Isolated Systems • A System that exchanges neither matter nor energy with its environment. • This cannot exist. • Possible exception? ? ?

How Systems are Linked • Every system consists of matter and energy …therefore are linked…. How?

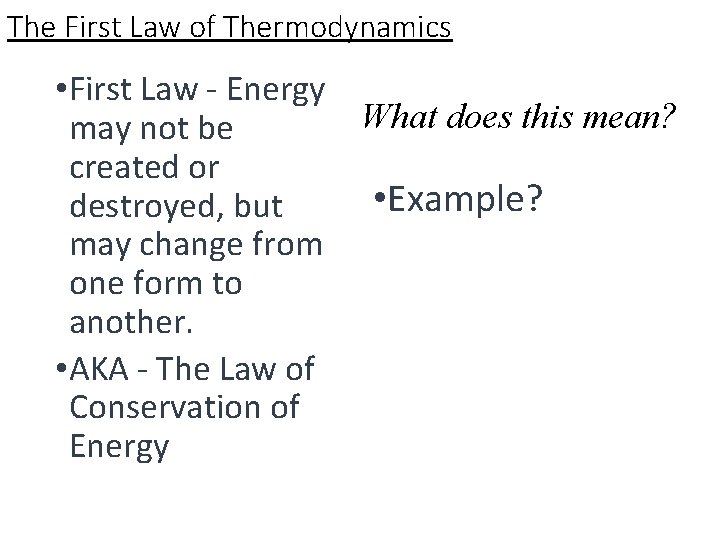
The First Law of Thermodynamics • First Law - Energy What does this mean? may not be created or • Example? destroyed, but may change from one form to another. • AKA - The Law of Conservation of Energy

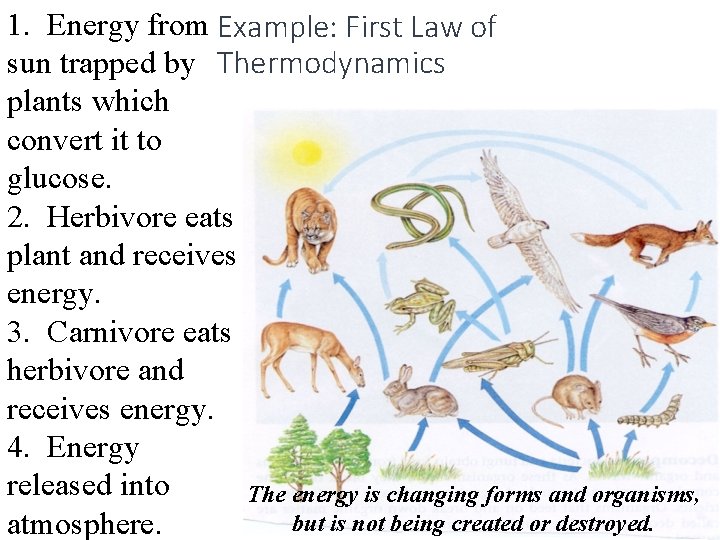
1. Energy from Example: First Law of sun trapped by Thermodynamics plants which convert it to glucose. 2. Herbivore eats plant and receives energy. 3. Carnivore eats herbivore and receives energy. 4. Energy released into The energy is changing forms and organisms, but is not being created or destroyed. atmosphere.

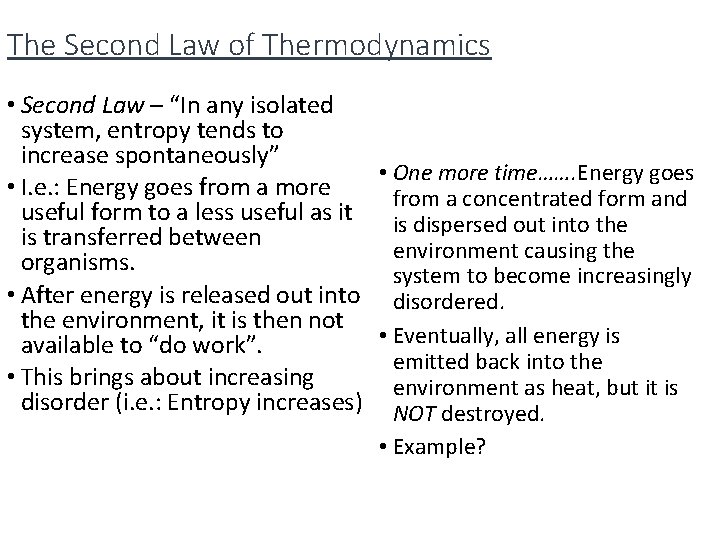
The Second Law of Thermodynamics • Second Law – “In any isolated system, entropy tends to increase spontaneously” • One more time……. Energy goes • I. e. : Energy goes from a more from a concentrated form and useful form to a less useful as it is dispersed out into the is transferred between environment causing the organisms. system to become increasingly • After energy is released out into disordered. the environment, it is then not • Eventually, all energy is available to “do work”. emitted back into the • This brings about increasing environment as heat, but it is disorder (i. e. : Entropy increases) NOT destroyed. • Example?

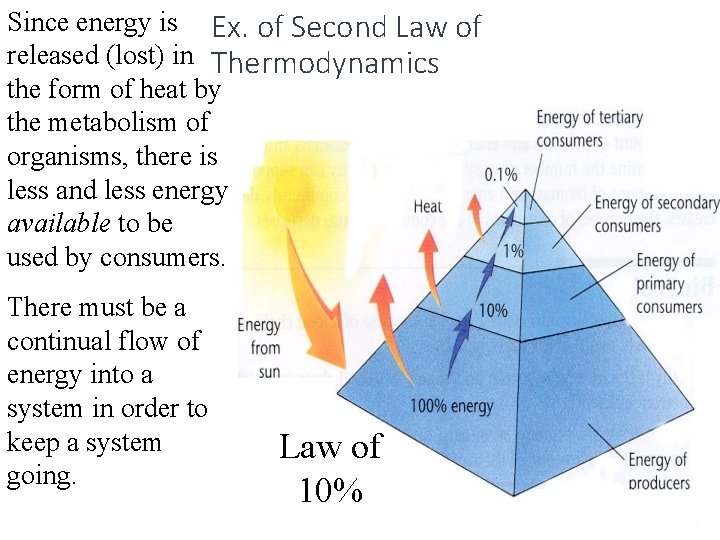
Since energy is Ex. of Second Law of released (lost) in Thermodynamics the form of heat by the metabolism of organisms, there is less and less energy available to be used by consumers. There must be a continual flow of energy into a system in order to keep a system going. Law of 10%

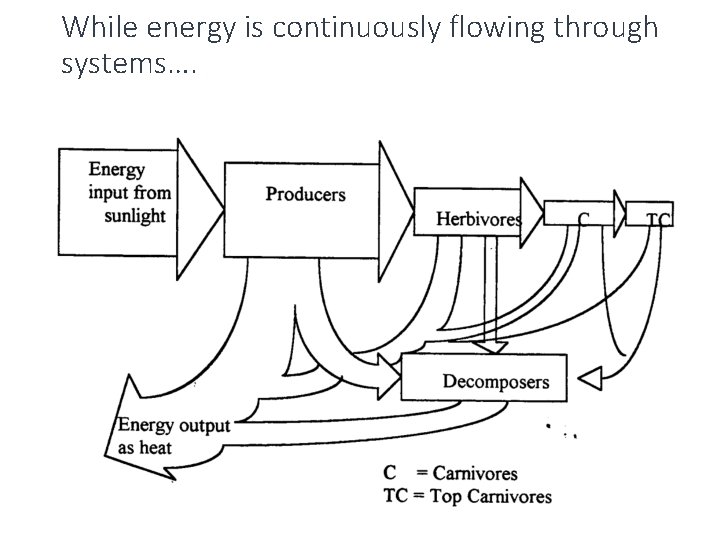
While energy is continuously flowing through systems….

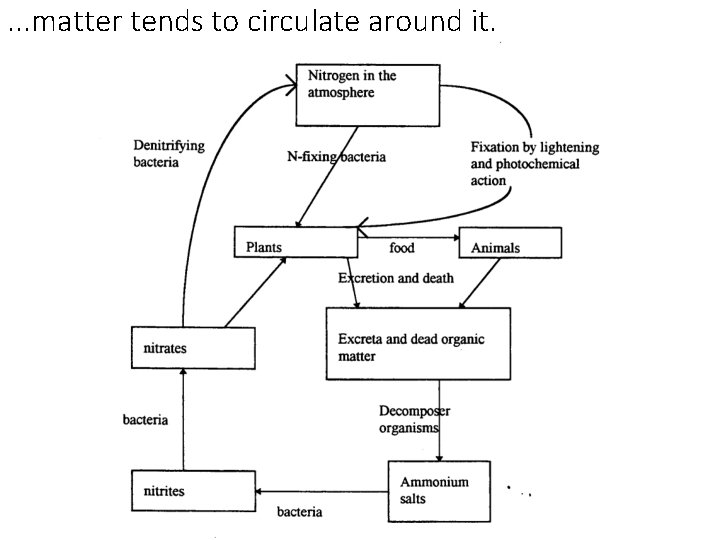
. . . matter tends to circulate around it.