Environmental Science 1 e SUSTAINING YOUR WORLD G














- Slides: 35

Environmental Science, 1 e SUSTAINING YOUR WORLD G. TYLER MILLER | SCOTT E. SPOOLMAN 12 Nonrenewable Energy Resources © 2017 Cengage Learning. All Rights Reserved.

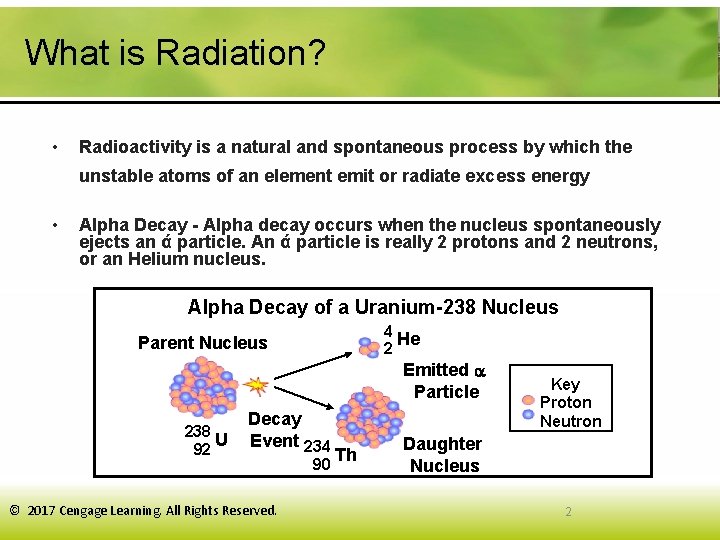
What is Radiation? • Radioactivity is a natural and spontaneous process by which the unstable atoms of an element emit or radiate excess energy • Alpha Decay - Alpha decay occurs when the nucleus spontaneously ejects an ά particle. An ά particle is really 2 protons and 2 neutrons, or an Helium nucleus. Alpha Decay of a Uranium-238 Nucleus 4 He 2 Parent Nucleus Emitted Particle 238 U 92 Decay Event 234 © 2017 Cengage Learning. All Rights Reserved. 90 Th Key Proton Neutron Daughter Nucleus 2

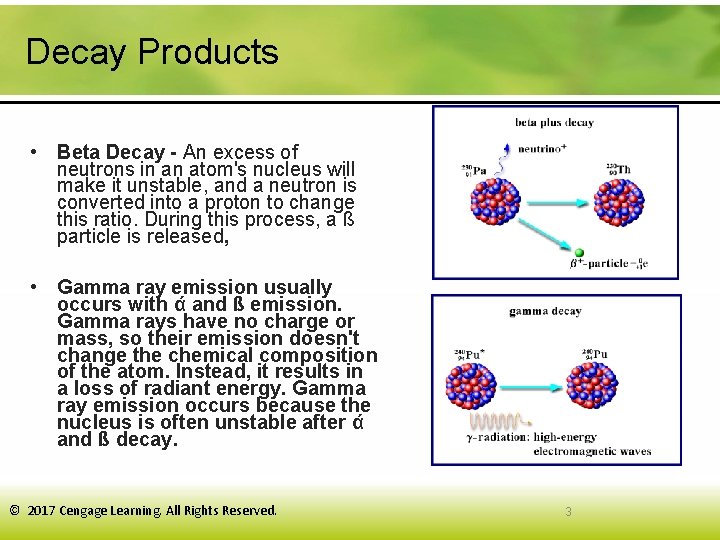
Decay Products • Beta Decay - An excess of neutrons in an atom's nucleus will make it unstable, and a neutron is converted into a proton to change this ratio. During this process, a ß particle is released, • Gamma ray emission usually occurs with ά and ß emission. Gamma rays have no charge or mass, so their emission doesn't change the chemical composition of the atom. Instead, it results in a loss of radiant energy. Gamma ray emission occurs because the nucleus is often unstable after ά and ß decay. © 2017 Cengage Learning. All Rights Reserved. 3

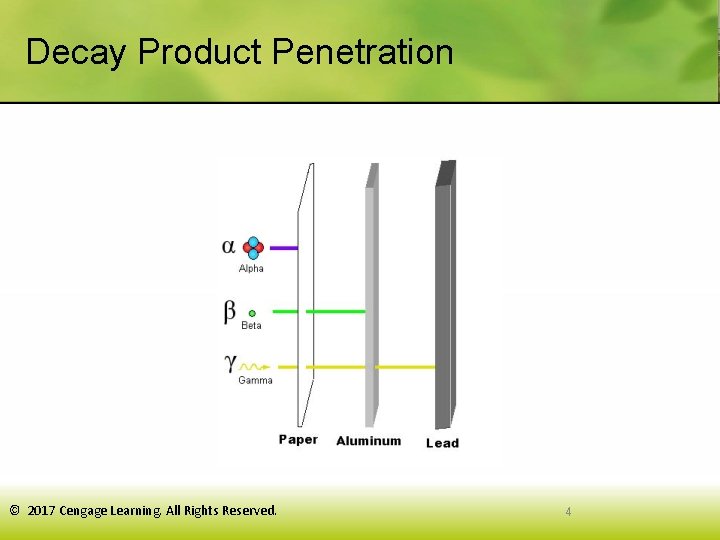
Decay Product Penetration © 2017 Cengage Learning. All Rights Reserved. 4

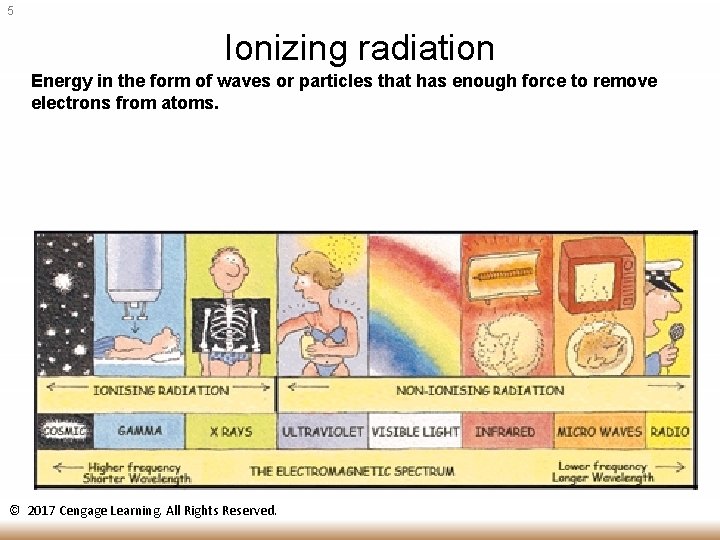
5 Ionizing radiation Energy in the form of waves or particles that has enough force to remove electrons from atoms. © 2017 Cengage Learning. All Rights Reserved.

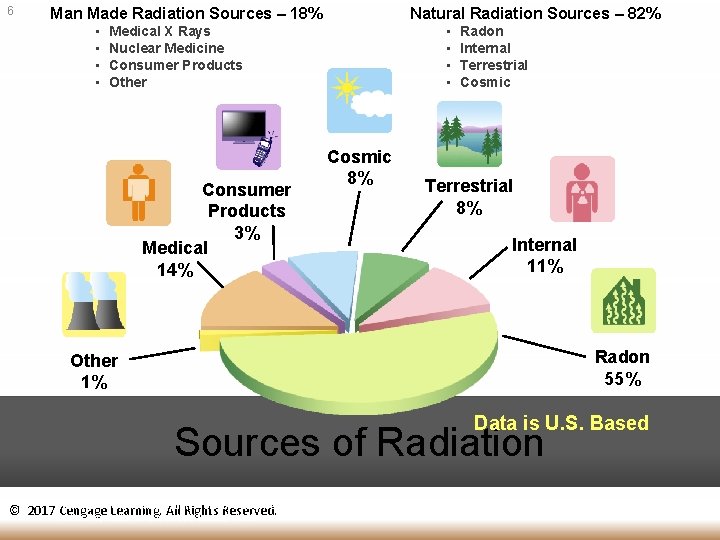
6 Man Made Radiation Sources – 18% • • Natural Radiation Sources – 82% • • Medical X Rays Nuclear Medicine Consumer Products Other Consumer Products 3% Medical 14% Cosmic 8% Radon Internal Terrestrial Cosmic Terrestrial 8% Internal 11% Radon 55% Other 1% Data is U. S. Based Sources of Radiation National Council on Radiation Protection and Measurements (NCRP) Report No. 93, “Ionizing Radiation Exposure © 2017 Cengage Learning. All Rights Reserved. of the Population of the United States, “ 1987

7 How to Detect Radiation • Geiger counter –Senses extremely tiny electrical impulses caused by radiation © 2017 Cengage Learning. All Rights Reserved.

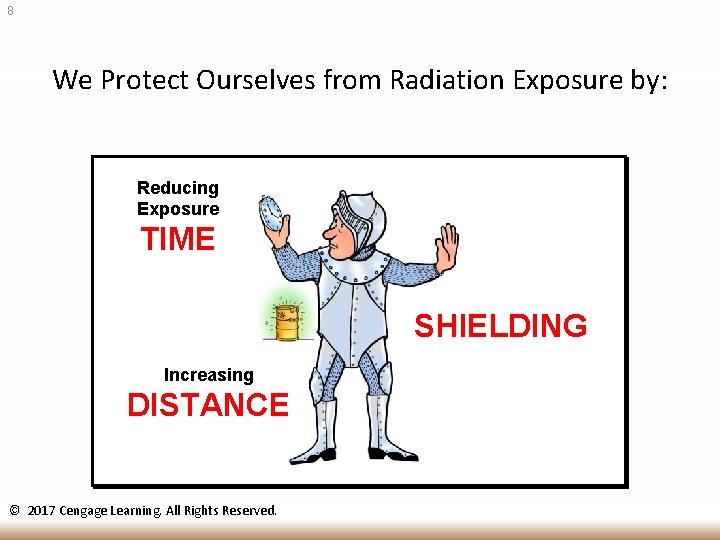
8 We Protect Ourselves from Radiation Exposure by: Reducing Exposure TIME SHIELDING Increasing DISTANCE © 2017 Cengage Learning. All Rights Reserved.

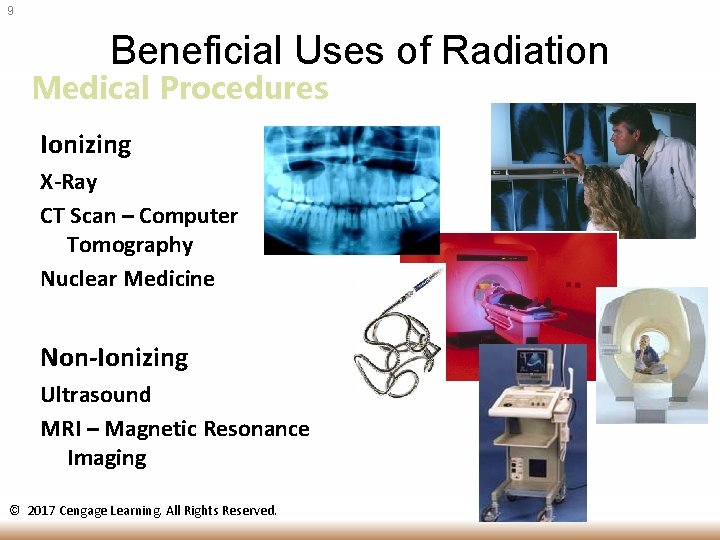
9 Beneficial Uses of Radiation Medical Procedures Ionizing X-Ray CT Scan – Computer Tomography Nuclear Medicine Non-Ionizing Ultrasound MRI – Magnetic Resonance Imaging © 2017 Cengage Learning. All Rights Reserved.

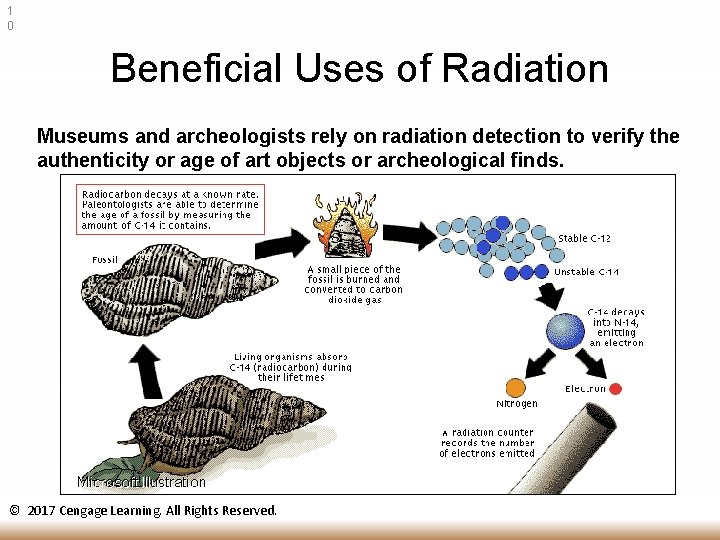
1 0 Beneficial Uses of Radiation Museums and archeologists rely on radiation detection to verify the authenticity or age of art objects or archeological finds. © 2017 Cengage Learning. All Rights Reserved.

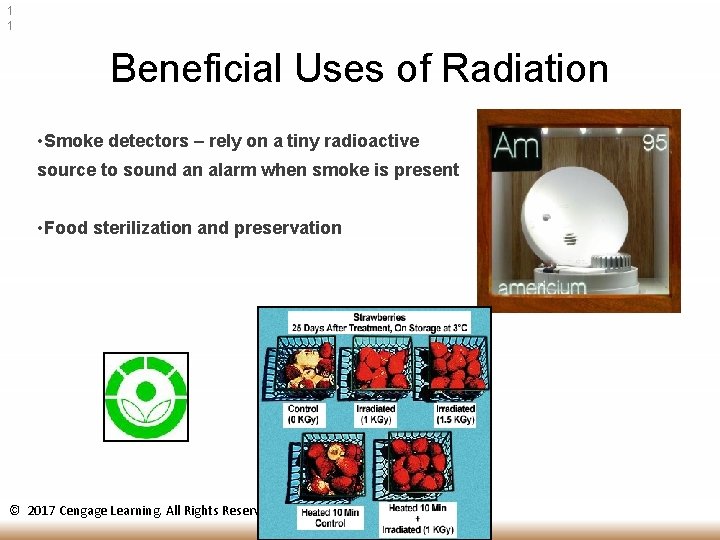
1 1 Beneficial Uses of Radiation • Smoke detectors – rely on a tiny radioactive source to sound an alarm when smoke is present • Food sterilization and preservation © 2017 Cengage Learning. All Rights Reserved.

Nuclear energy starts with a split. Energy is stored in the nuclei of atoms. Unstable atoms release energy all the time. But big energy happens when their nuclei split. • We call this split fission. © 2017 Cengage Learning. All Rights Reserved. 12

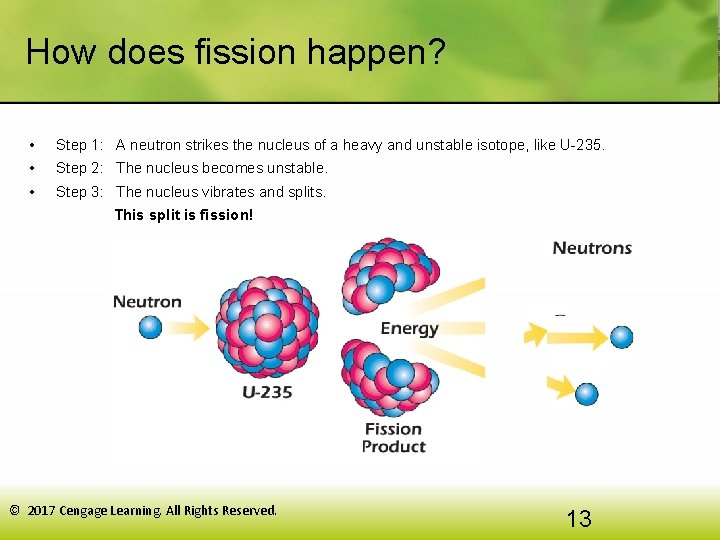
How does fission happen? • Step 1: A neutron strikes the nucleus of a heavy and unstable isotope, like U-235. • Step 2: The nucleus becomes unstable. • Step 3: The nucleus vibrates and splits. This split is fission! © 2017 Cengage Learning. All Rights Reserved. 13

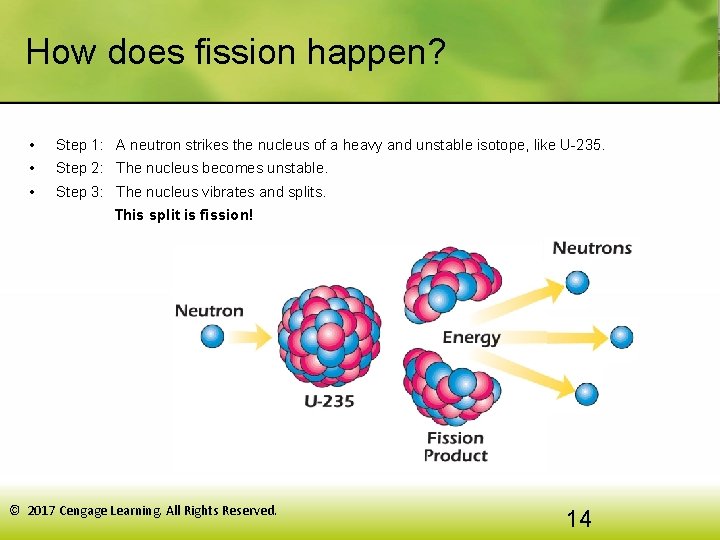
How does fission happen? • Step 1: A neutron strikes the nucleus of a heavy and unstable isotope, like U-235. • Step 2: The nucleus becomes unstable. • Step 3: The nucleus vibrates and splits. This split is fission! © 2017 Cengage Learning. All Rights Reserved. 14

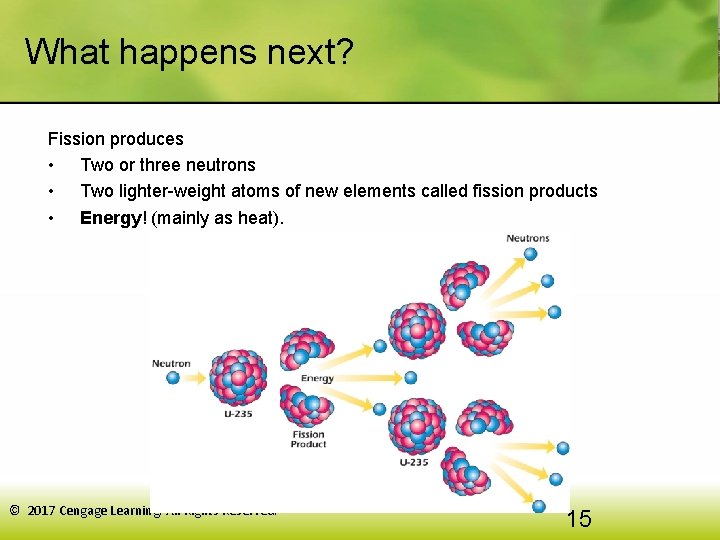
What happens next? Fission produces • Two or three neutrons • Two lighter-weight atoms of new elements called fission products • Energy! (mainly as heat). © 2017 Cengage Learning. All Rights Reserved. 15

What happens next keeps happening. In a nuclear chain reaction, fission releases more neutrons, which split more atoms. We call it a chain reaction because it keeps happening! Here is how two science classes demonstrated a chain reaction: http: //www. youtube. com/watch? v=0 v 8 i 4 v 1 mie. U&feature=player_embedded http: //www. youtube. com/watch? v=FQGtpo 2 IUx. A&feature=fvwrel © 2017 Cengage Learning. All Rights Reserved. 16

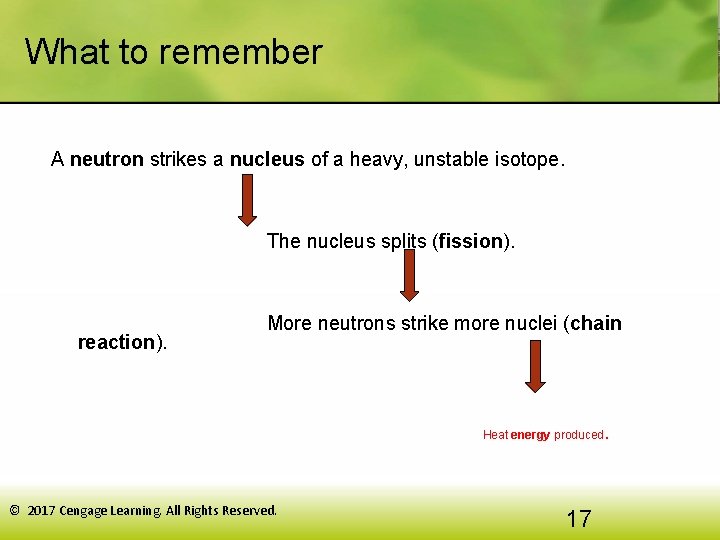
What to remember A neutron strikes a nucleus of a heavy, unstable isotope. The nucleus splits (fission). reaction). More neutrons strike more nuclei (chain . Heat energy produced © 2017 Cengage Learning. All Rights Reserved. 17

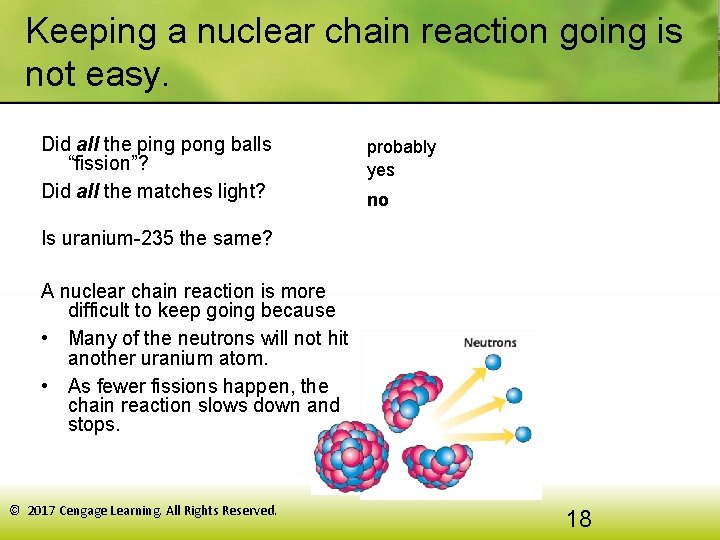
Keeping a nuclear chain reaction going is not easy. Did all the ping pong balls “fission”? Did all the matches light? probably yes no Is uranium-235 the same? A nuclear chain reaction is more difficult to keep going because • Many of the neutrons will not hit another uranium atom. • As fewer fissions happen, the chain reaction slows down and stops. © 2017 Cengage Learning. All Rights Reserved. 18

Uranium • URANIUM is a slightly radioactive metal that occurs throughout the earth's crust. • It is about 500 times more abundant than gold and about as common as tin. • It is present in most rocks and soils as well as in many rivers and in sea water. • Most of the radioactivity associated with uranium in nature is due to other materials derived from it by radioactive decay processes, and which are left behind in mining and milling. • Economically feasible deposits of the ore, pitchblende, U 3 O 8, range from 0. 1% to 20% U 3 O 8. © 2017 Cengage Learning. All Rights Reserved.

12. 3 What Are the Advantages and Disadvantages of Using Nuclear Power? • Nuclear power has a low environmental impact, but its use has been limited by: – A low net energy, high costs, fear of accidents, and long-lived radioactive wastes – Its role in spreading nuclear weapons technology © 2017 Cengage Learning. All Rights Reserved.

How Does a Nuclear Fission Reactor Work? • Controlled nuclear fission: a neutron is used to split a large nucleus into two or more smaller nuclei – Carried out in light-water reactor – Fueled by uranium ore – Enormous amount of energy released in short time – Heat used to generate electricity © 2017 Cengage Learning. All Rights Reserved.

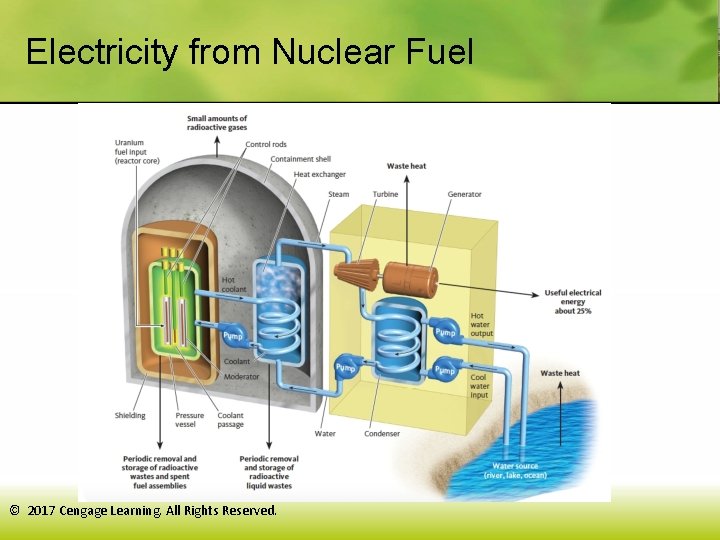
Electricity from Nuclear Fuel © 2017 Cengage Learning. All Rights Reserved.

Steps of the Nuclear Fuel Cycle • • • Mine the uranium. Process the uranium to make the fuel. Use it in the reactor. Safely store the radioactive waste. Decommission the reactor. © 2017 Cengage Learning. All Rights Reserved.

What is the fuel at a nuclear power plant? A nuclear power plant uses uranium for fuel. Uranium …. . • Is a dense, heavy metal • Consists of atoms that hold a lot of energy in their nuclei • Is found in ordinary rocks and soil around the world. Uranium ore is mined as rocks like this one. © 2017 Cengage Learning. All Rights Reserved. 24

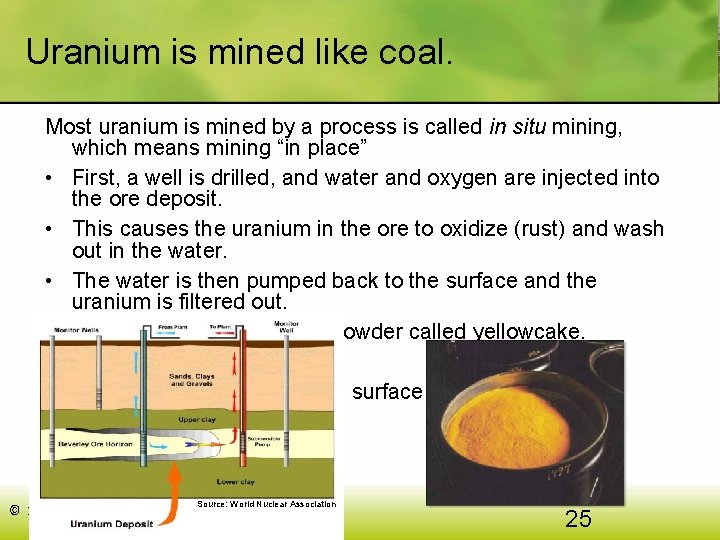
Uranium is mined like coal. Most uranium is mined by a process is called in situ mining, which means mining “in place” • First, a well is drilled, and water and oxygen are injected into the ore deposit. • This causes the uranium in the ore to oxidize (rust) and wash out in the water. • The water is then pumped back to the surface and the uranium is filtered out. • What’s left is a dry, yellow powder called yellowcake. Some uranium is also mined in surface and deep mines. Source: World Nuclear Association © 2017 Cengage Learning. All Rights Reserved. 25

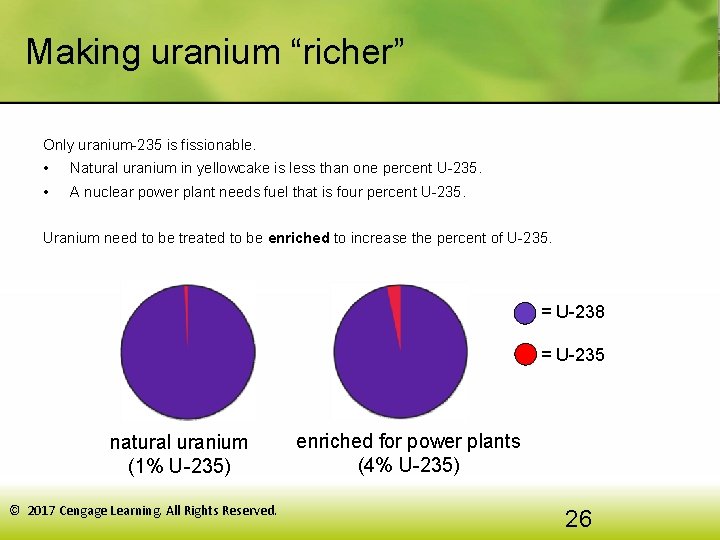
Making uranium “richer” Only uranium-235 is fissionable. • Natural uranium in yellowcake is less than one percent U-235. • A nuclear power plant needs fuel that is four percent U-235. Uranium need to be treated to be enriched to increase the percent of U-235. = U-238 = U-235 natural uranium (1% U-235) © 2017 Cengage Learning. All Rights Reserved. enriched for power plants (4% U-235) 26

UF 6 is a solid, gas, and liquid. • • Before it can be enriched, yellowcake is converted into uranium hexafluoride (UF 6). At room temperature, UF 6 changes into solid crystals that look like this: • When the crystals are heated, they become a gas. © 2017 Cengage Learning. All Rights Reserved. 27

Ready for a nuclear power plant Enriched uranium for a power plant has about 4 percent U-235. It is • • Made into a ceramic material Formed into small fuel pellets Stacked in rods that are grouped into assemblies Sent to nuclear power plants. The fuel lasts for 3 years. © 2017 Cengage Learning. All Rights Reserved. 28

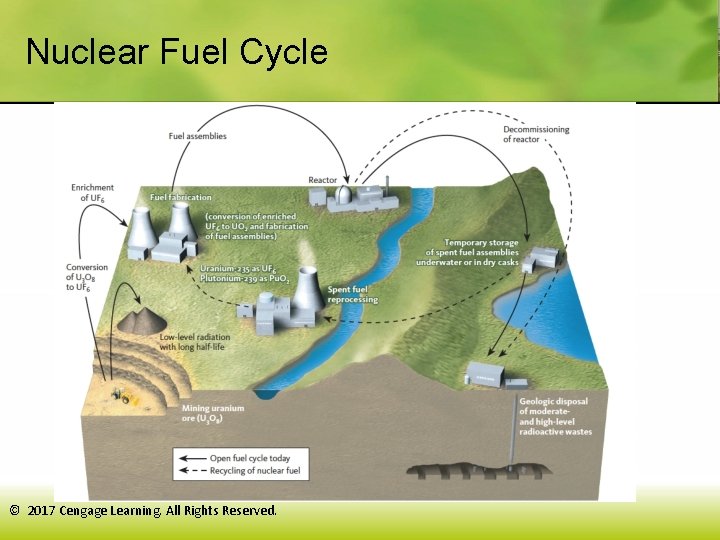
Nuclear Fuel Cycle © 2017 Cengage Learning. All Rights Reserved.

Dealing with Radioactive Nuclear Wastes • High-level radioactive wastes – Cause cancer – Must be stored safely for 10, 000– 240, 000 years – Too hot and radioactive to throw away – Water-filled pools and dry cask storage (only last fraction of time needed for safe disposal) – Reprocessing an option but very costly – Deep burial: safest but most costly option – Still no acceptable method of handling © 2017 Cengage Learning. All Rights Reserved.

Decommissioning Nuclear Power Plants • Dealing with old nuclear power plants: – Decommission or retire power plant – Dismantle plant and safely store the radioactive materials – Enclose plant behind a physical barrier with fulltime security until a storage facility has been built – Enclose the plant in a tomb • Monitor this for thousands of years © 2017 Cengage Learning. All Rights Reserved.

Nuclear Accidents Spread Uncertainty • Loss of coolant water causes a meltdown in the reactor core. – Explosions, release of radioactivity into environment – Three Mile Island (United States) – Chernobyl (Ukraine) – Fukushima Daiichi (Japan) © 2017 Cengage Learning. All Rights Reserved.

Experts Disagree about the Future of Nuclear Power • Proponents of nuclear power: – No CO 2 emissions during plant operation – Research potentially cheaper/safer reactors – Develop nuclear fusion – Continue subsidies • Opponents of nuclear power: – Risk of accidents – Damage to environment – Nuclear weapons © 2017 Cengage Learning. All Rights Reserved.

Is Nuclear Fusion the Answer? • Fusion – Two isotopes fused together to form a heavier nucleus – Releases energy • Technology is very difficult to develop © 2017 Cengage Learning. All Rights Reserved.

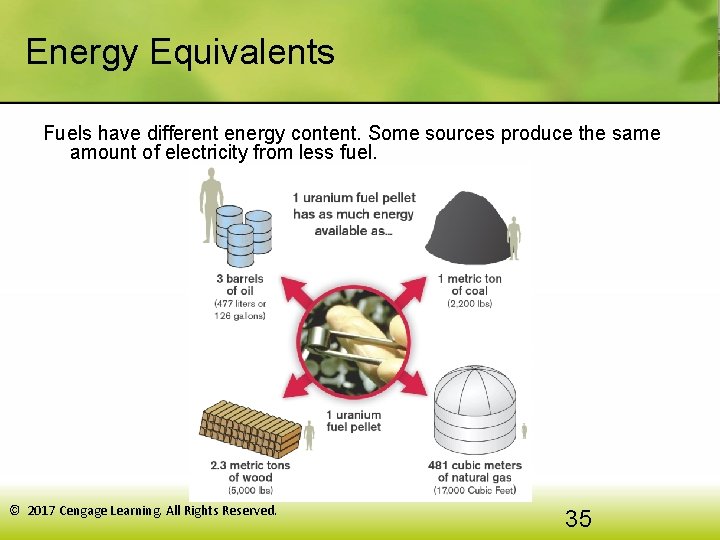
Energy Equivalents Fuels have different energy content. Some sources produce the same amount of electricity from less fuel. © 2017 Cengage Learning. All Rights Reserved. 35