ENVIRONMENTAL MICROBIOLOGY LABORATORY MANUALPREPARED FOR ENVIRONMENTAL MICROBIOLOGY IV

ENVIRONMENTAL MICROBIOLOGY -LABORATORY MANUALPREPARED FOR ENVIRONMENTAL MICROBIOLOGY IV BIOCHEMICAL ACTIVITY OF MICROORGANISM ENVIRONMENTAL MANAGEMENT TECHNOLOGY FACULTY OF CIVIL AND ENVIRONMENTAL ENGINEERING ITB, 2010
Terms Metabolism Anabolism Catalytic Catalyst Enzyme Apoenzyme Holoenzyme Coenzym Endoenzyme Exoenzyme Enzyme activity Enzyme system Substrate Active Site Oxidation Reduction Hydrolysis Glycolysis Fermentation Aerobic respiration Anaerobic respiration Peptide bonding Peptonization Starch Carbohydrate Amino acid Lipid Fatty acid Protein Casein Pyruvic acid ATP, ADP
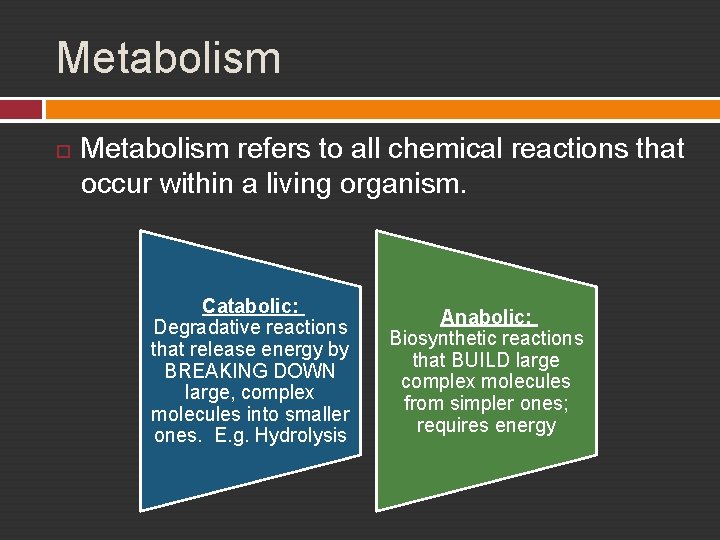
Metabolism refers to all chemical reactions that occur within a living organism. Catabolic: Degradative reactions that release energy by BREAKING DOWN large, complex molecules into smaller ones. E. g. Hydrolysis Anabolic: Biosynthetic reactions that BUILD large complex molecules from simpler ones; requires energy
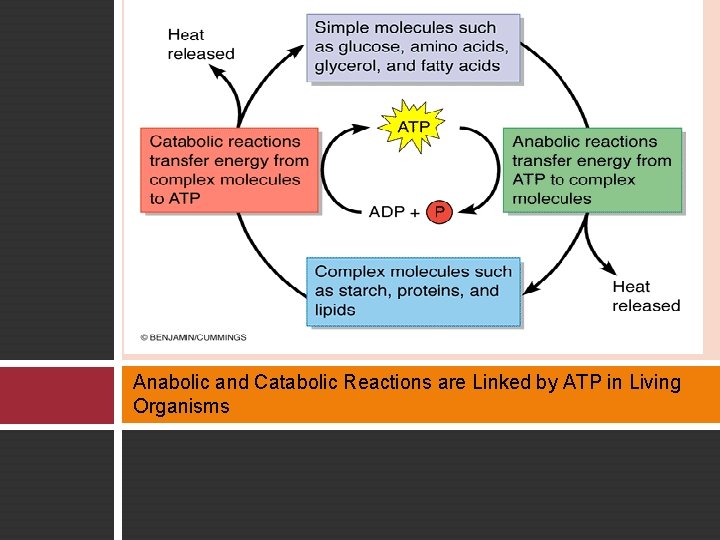
Anabolic and Catabolic Reactions are Linked by ATP in Living Organisms
Catalyst = an agent that accelerates chemical reaction without itself being destroyed or used up ENZYME

Enzyme Organic catalyst (elaborated by living cell) Protein mollecular Thermolabile (denaturated by heat) Precipitated by ethanol and high concentration inorganic salts Non-dialyzable (does not go through semipermeable membrane)
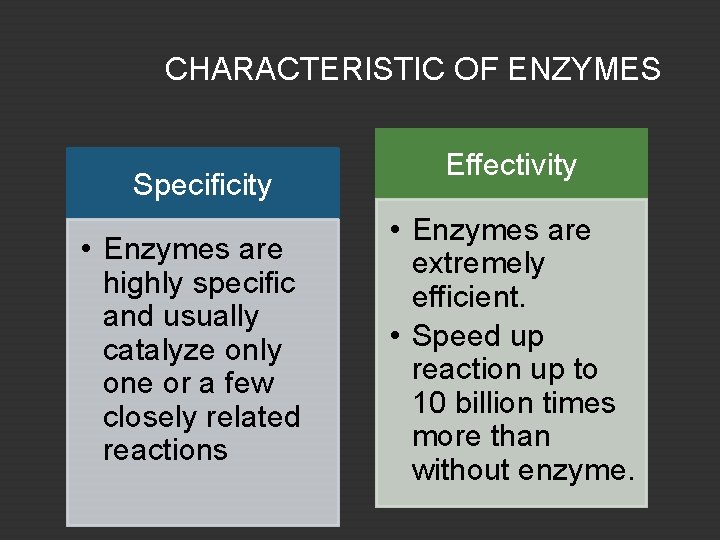
CHARACTERISTIC OF ENZYMES Specificity • Enzymes are highly specific and usually catalyze only one or a few closely related reactions Effectivity • Enzymes are extremely efficient. • Speed up reaction up to 10 billion times more than without enzyme.
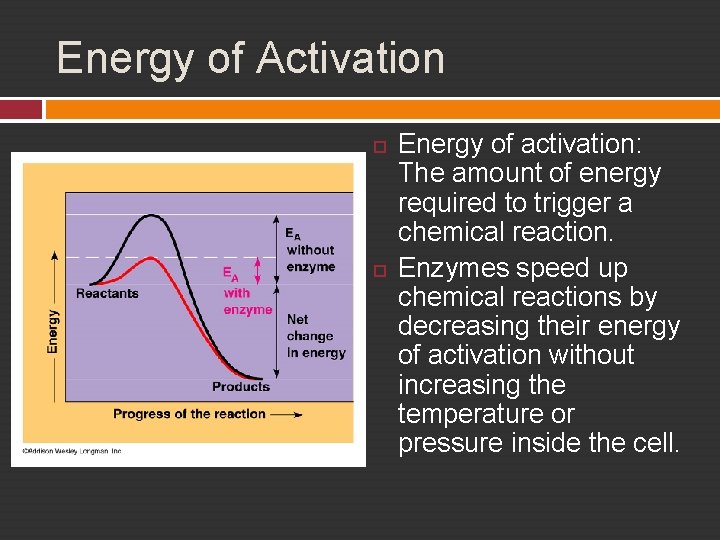
Energy of Activation Energy of activation: The amount of energy required to trigger a chemical reaction. Enzymes speed up chemical reactions by decreasing their energy of activation without increasing the temperature or pressure inside the cell.
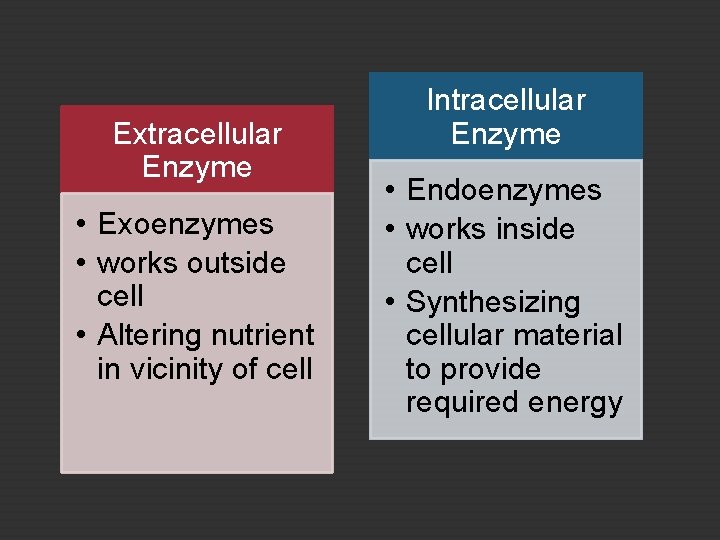
Extracellular Enzyme • Exoenzymes • works outside cell • Altering nutrient in vicinity of cell Intracellular Enzyme • Endoenzymes • works inside cell • Synthesizing cellular material to provide required energy
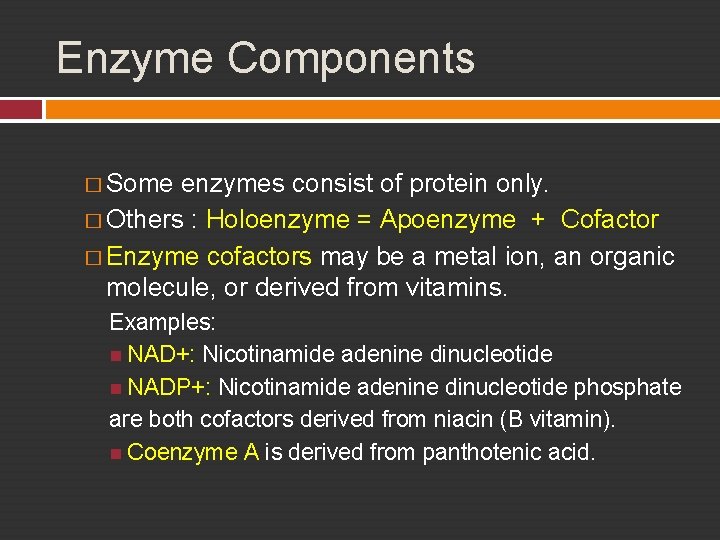
Enzyme Components � Some enzymes consist of protein only. � Others : Holoenzyme = Apoenzyme + Cofactor � Enzyme cofactors may be a metal ion, an organic molecule, or derived from vitamins. Examples: NAD+: Nicotinamide adenine dinucleotide NADP+: Nicotinamide adenine dinucleotide phosphate are both cofactors derived from niacin (B vitamin). Coenzyme A is derived from panthotenic acid.
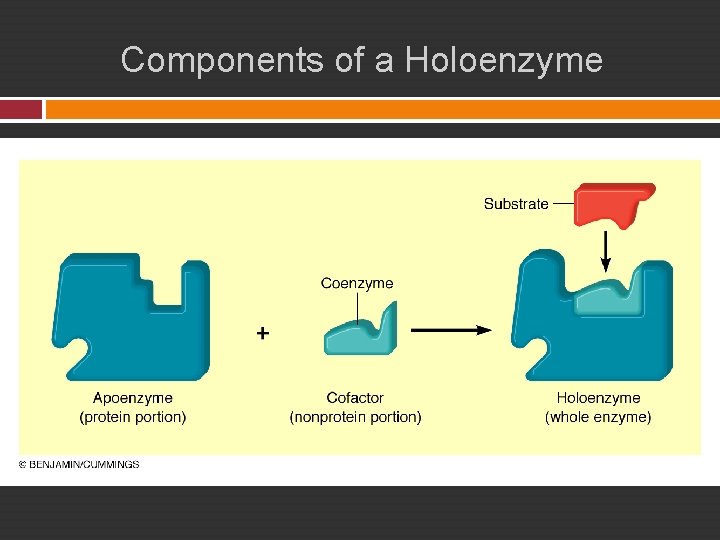
Components of a Holoenzyme
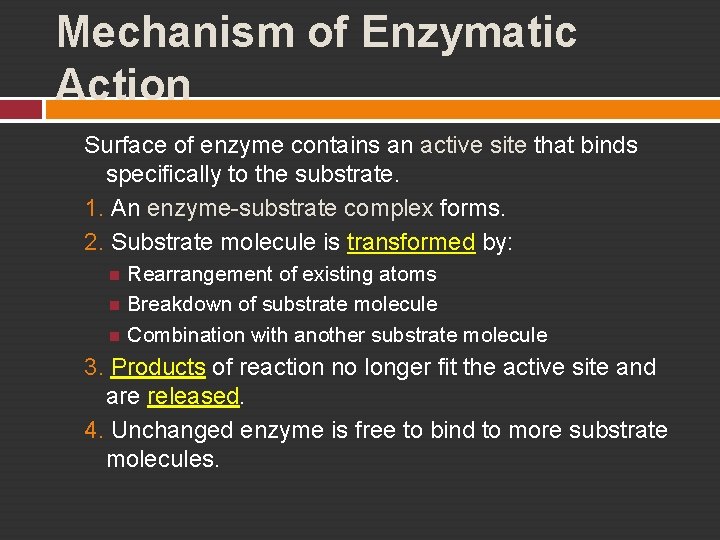
Mechanism of Enzymatic Action Surface of enzyme contains an active site that binds specifically to the substrate. 1. An enzyme-substrate complex forms. 2. Substrate molecule is transformed by: Rearrangement of existing atoms Breakdown of substrate molecule Combination with another substrate molecule 3. Products of reaction no longer fit the active site and are released. 4. Unchanged enzyme is free to bind to more substrate molecules.
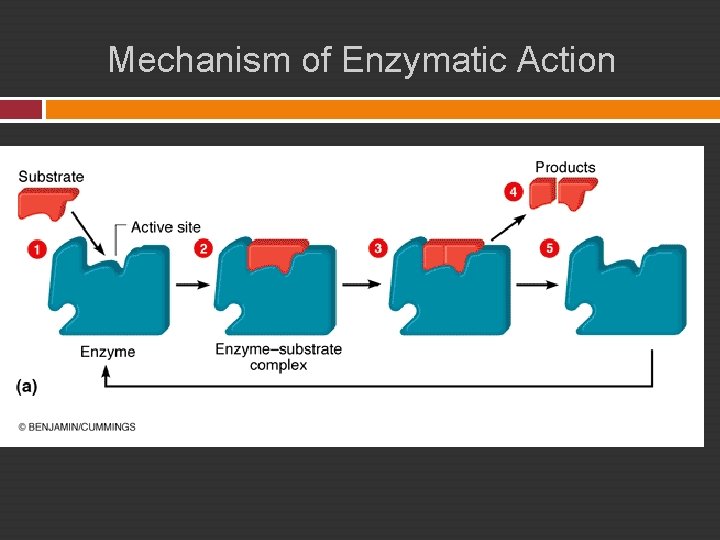
Mechanism of Enzymatic Action
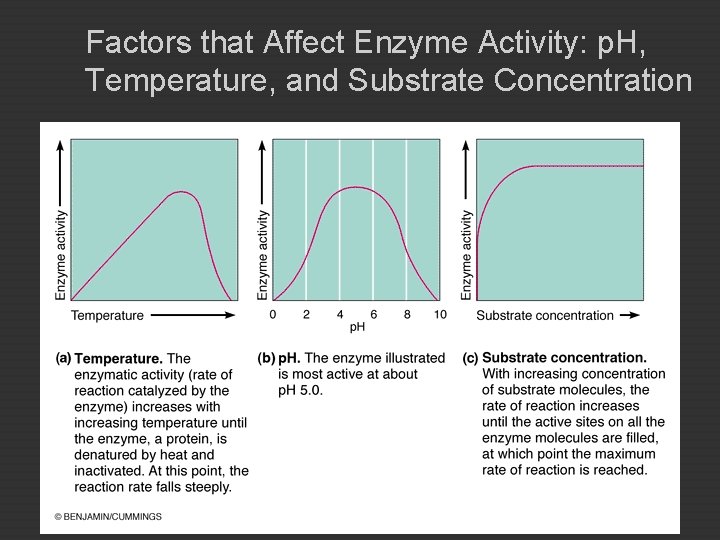
Factors that Affect Enzyme Activity: p. H, Temperature, and Substrate Concentration
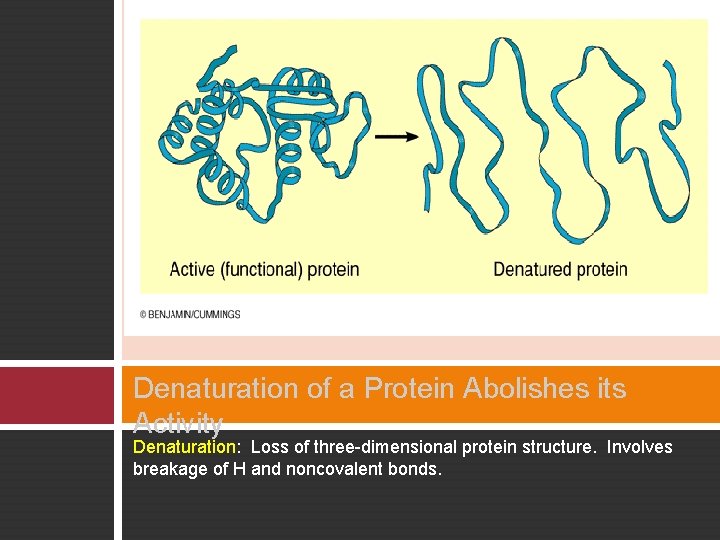
Denaturation of a Protein Abolishes its Activity Denaturation: Loss of three-dimensional protein structure. Involves breakage of H and noncovalent bonds.
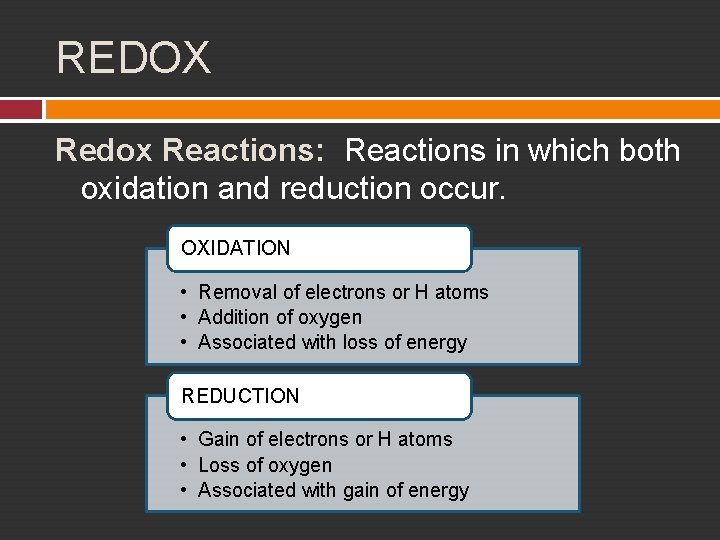
REDOX Redox Reactions: Reactions in which both oxidation and reduction occur. OXIDATION • Removal of electrons or H atoms • Addition of oxygen • Associated with loss of energy REDUCTION • Gain of electrons or H atoms • Loss of oxygen • Associated with gain of energy
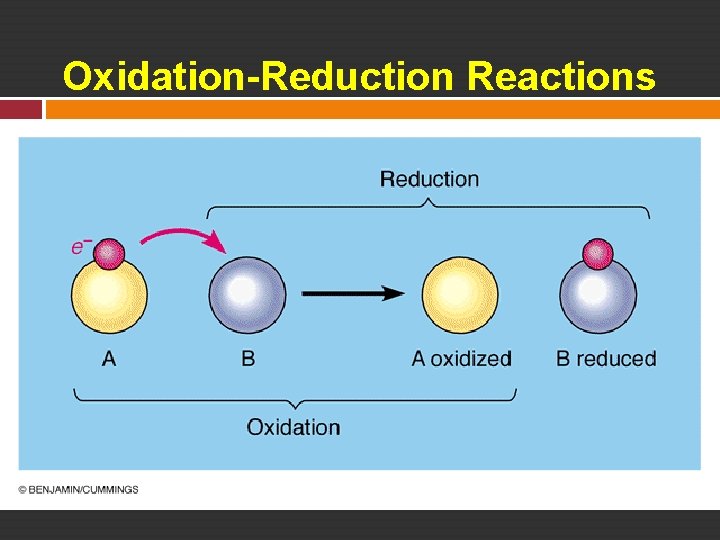
Oxidation-Reduction Reactions
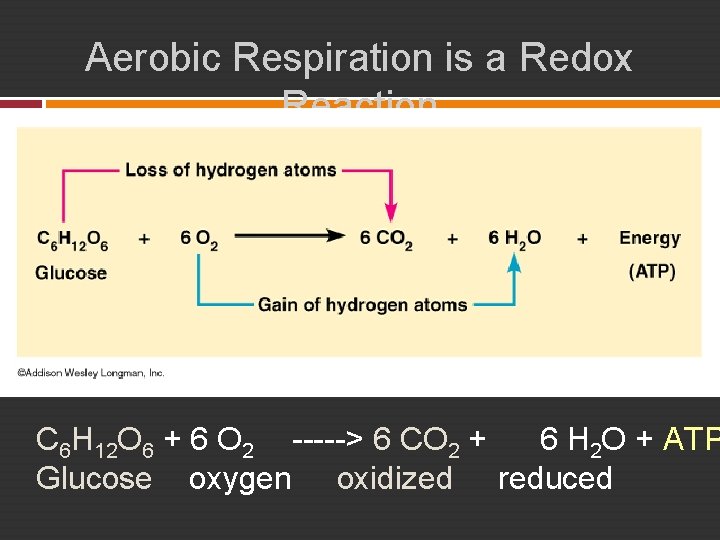
Aerobic Respiration is a Redox Reaction C 6 H 12 O 6 + 6 O 2 -----> 6 CO 2 + 6 H 2 O + ATP Glucose oxygen oxidized reduced
Hydrolysis is a chemical reaction during which molecules of water (H 2 O) are split into hydrogen cations (H+) (conventionally referred to as protons) and hydroxide anions (OH−) in the process of a chemical mechanism.
Carbohydrate Catabolism u u u Most microorganisms use glucose or other carbohydrates as their primary source of energy. Lipids and proteins are also used as energy sources. Two general processes are used to obtain energy from glucose: u cellular respiration u fermentation
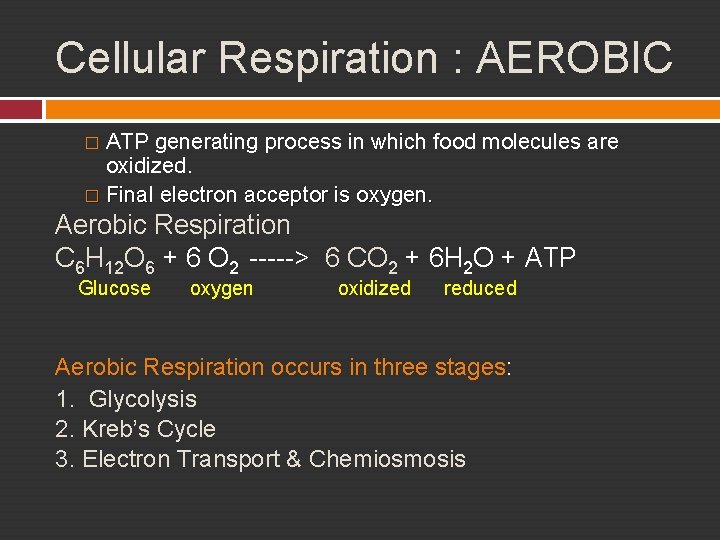
Cellular Respiration : AEROBIC ATP generating process in which food molecules are oxidized. � Final electron acceptor is oxygen. � Aerobic Respiration C 6 H 12 O 6 + 6 O 2 -----> 6 CO 2 + 6 H 2 O + ATP Glucose oxygen oxidized reduced Aerobic Respiration occurs in three stages: 1. Glycolysis 2. Kreb’s Cycle 3. Electron Transport & Chemiosmosis
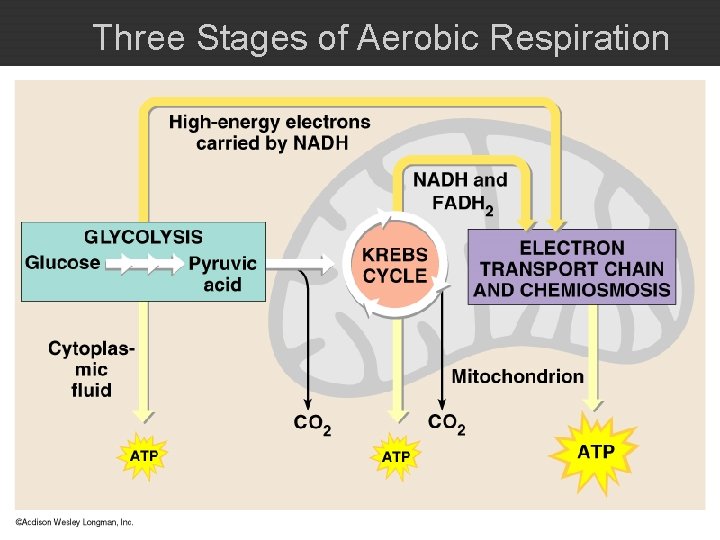
Three Stages of Aerobic Respiration
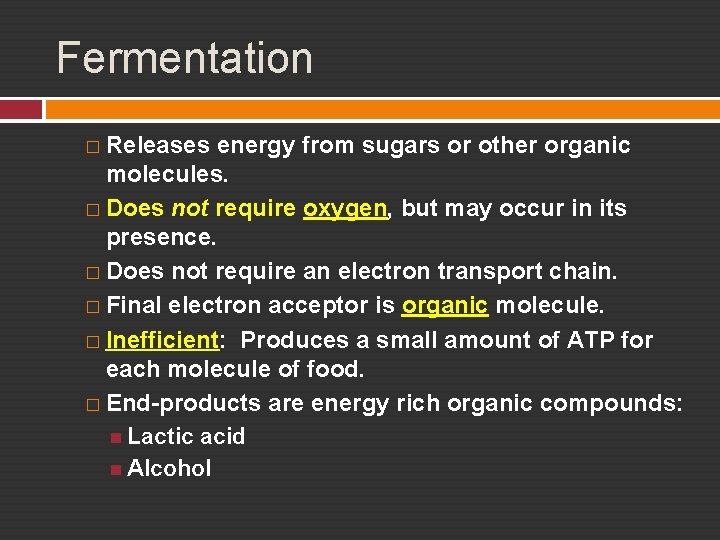
Fermentation � Releases energy from sugars or other organic molecules. � Does not require oxygen, but may occur in its presence. � Does not require an electron transport chain. � Final electron acceptor is organic molecule. � Inefficient: Produces a small amount of ATP for each molecule of food. � End-products are energy rich organic compounds: Lactic acid Alcohol
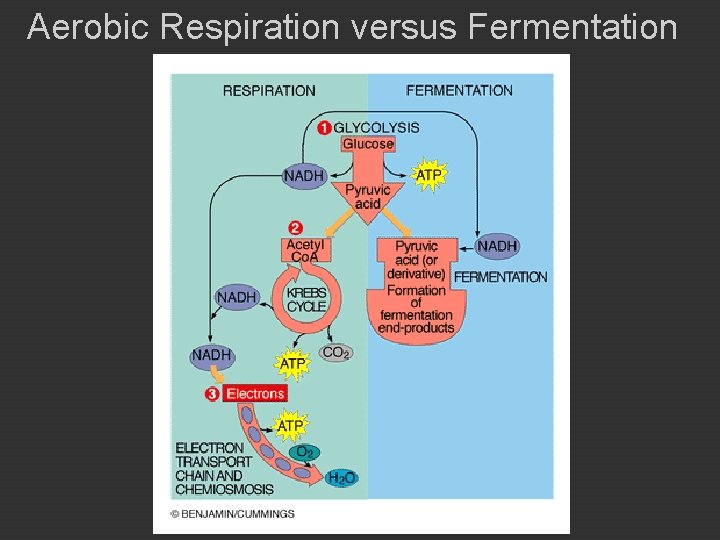
Aerobic Respiration versus Fermentation
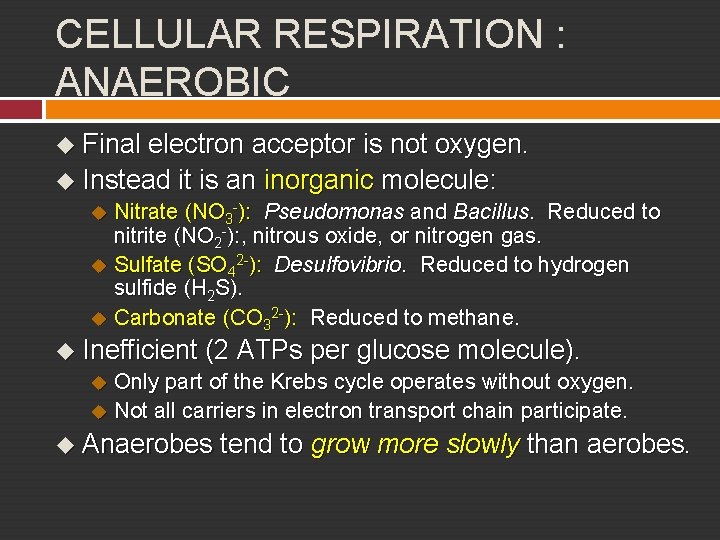
CELLULAR RESPIRATION : ANAEROBIC u Final electron acceptor is not oxygen. u Instead it is an inorganic molecule: u Nitrate (NO 3 -): Pseudomonas and Bacillus. Reduced to nitrite (NO 2 -): , nitrous oxide, or nitrogen gas. u Sulfate (SO 42 -): Desulfovibrio. Reduced to hydrogen sulfide (H 2 S). u Carbonate (CO 32 -): Reduced to methane. u Inefficient (2 ATPs per glucose molecule). u Only part of the Krebs cycle operates without oxygen. u Not all carriers in electron transport chain participate. u Anaerobes tend to grow more slowly than aerobes.

EXPERIMENTS
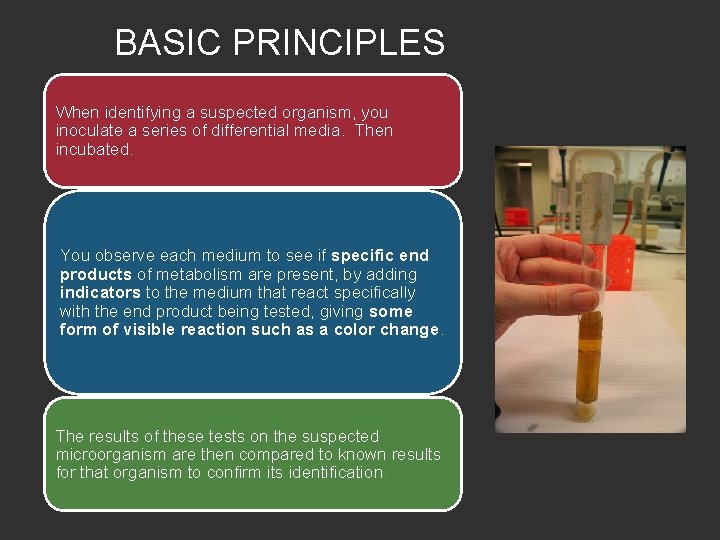
BASIC PRINCIPLES When identifying a suspected organism, you inoculate a series of differential media. Then incubated. You observe each medium to see if specific end products of metabolism are present, by adding indicators to the medium that react specifically with the end product being tested, giving some form of visible reaction such as a color change. The results of these tests on the suspected microorganism are then compared to known results for that organism to confirm its identification.
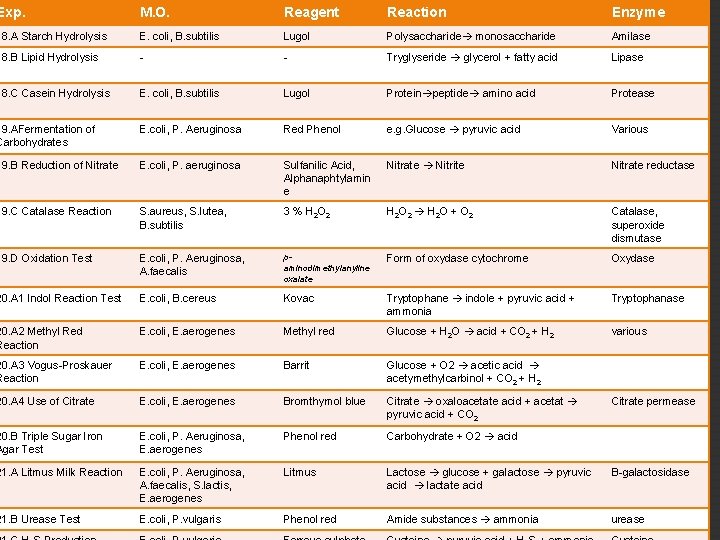
Exp. M. O. Reagent Reaction Enzyme 18. A Starch Hydrolysis E. coli, B. subtilis Lugol Polysaccharide monosaccharide Amilase 18. B Lipid Hydrolysis - - Tryglyseride glycerol + fatty acid Lipase 18. C Casein Hydrolysis E. coli, B. subtilis Lugol Protein peptide amino acid Protease 19. AFermentation of Carbohydrates E. coli, P. Aeruginosa Red Phenol e. g. Glucose pyruvic acid Various 19. B Reduction of Nitrate E. coli, P. aeruginosa Sulfanilic Acid, Alphanaphtylamin e Nitrate Nitrite Nitrate reductase 19. C Catalase Reaction S. aureus, S. lutea, B. subtilis 3 % H 2 O 2 H 2 O + O 2 Catalase, superoxide dismutase 19. D Oxidation Test E. coli, P. Aeruginosa, A. faecalis paminodimethylanyline oxalate Form of oxydase cytochrome Oxydase 20. A 1 Indol Reaction Test E. coli, B. cereus Kovac Tryptophane indole + pyruvic acid + ammonia Tryptophanase 20. A 2 Methyl Red Reaction E. coli, E. aerogenes Methyl red Glucose + H 2 O acid + CO 2 + H 2 various 20. A 3 Vogus-Proskauer Reaction E. coli, E. aerogenes Barrit Glucose + O 2 acetic acid acetymethylcarbinol + CO 2 + H 2 20. A 4 Use of Citrate E. coli, E. aerogenes Bromthymol blue Citrate oxaloacetate acid + acetat pyruvic acid + CO 2 20. B Triple Sugar Iron Agar Test E. coli, P. Aeruginosa, E. aerogenes Phenol red Carbohydrate + O 2 acid 21. A Litmus Milk Reaction E. coli, P. Aeruginosa, A. faecalis, S. lactis, E. aerogenes Litmus Lactose glucose + galactose pyruvic acid lactate acid B-galactosidase 21. B Urease Test E. coli, P. vulgaris Phenol red Amide substances ammonia urease Citrate permease

Exp. 18 – Acivities of Extracelullar Enzymes Hydrolysis of Starch and Casein
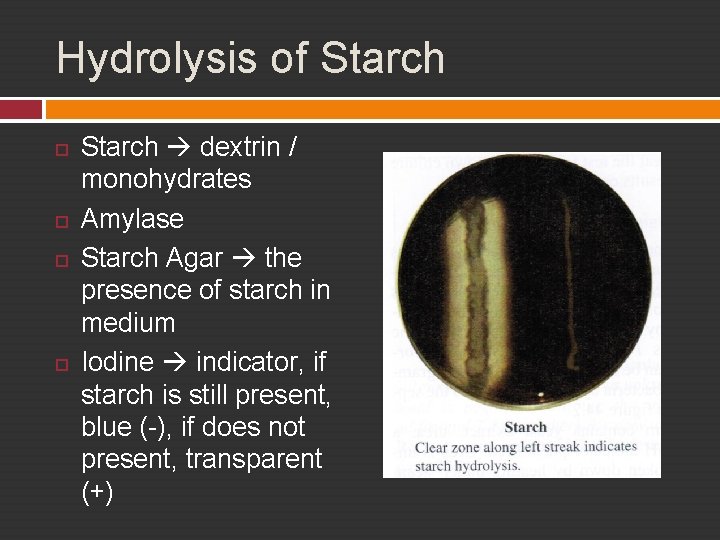
Hydrolysis of Starch dextrin / monohydrates Amylase Starch Agar the presence of starch in medium Iodine indicator, if starch is still present, blue (-), if does not present, transparent (+)
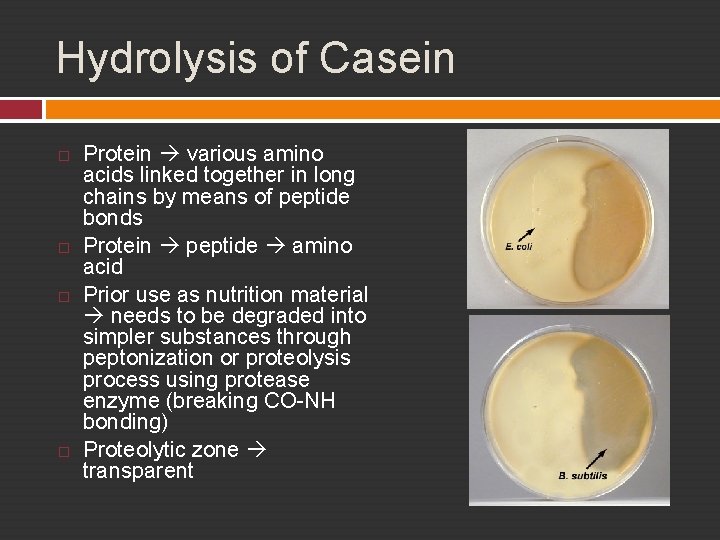
Hydrolysis of Casein Protein various amino acids linked together in long chains by means of peptide bonds Protein peptide amino acid Prior use as nutrition material needs to be degraded into simpler substances through peptonization or proteolysis process using protease enzyme (breaking CO-NH bonding) Proteolytic zone transparent
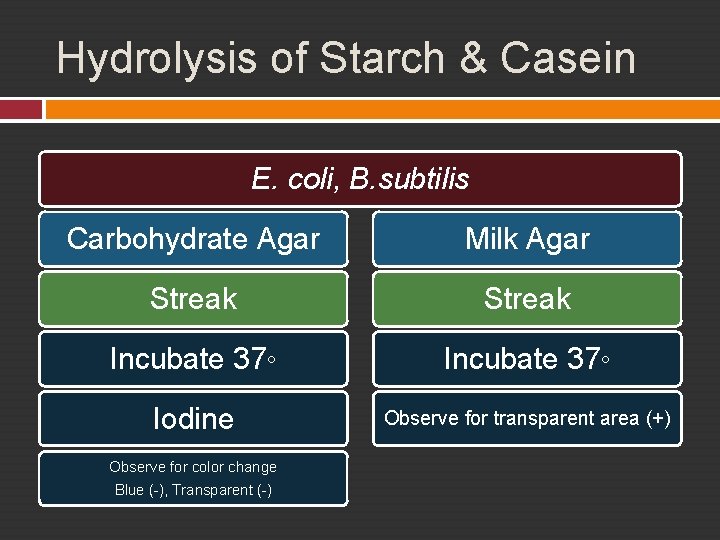
Hydrolysis of Starch & Casein E. coli, B. subtilis Carbohydrate Agar Milk Agar Streak Incubate 37◦ Iodine Observe for transparent area (+) Observe for color change Blue (-), Transparent (-)

Exp. 19 – Acivities of Intracelullar Enzymes : Fermentation Test and Oxidation 19. A. Fermentation of Carbohydrates 19. B. Reduction of Nitrate 19. C. Catalase Reaction 19. D. Oxidation Test

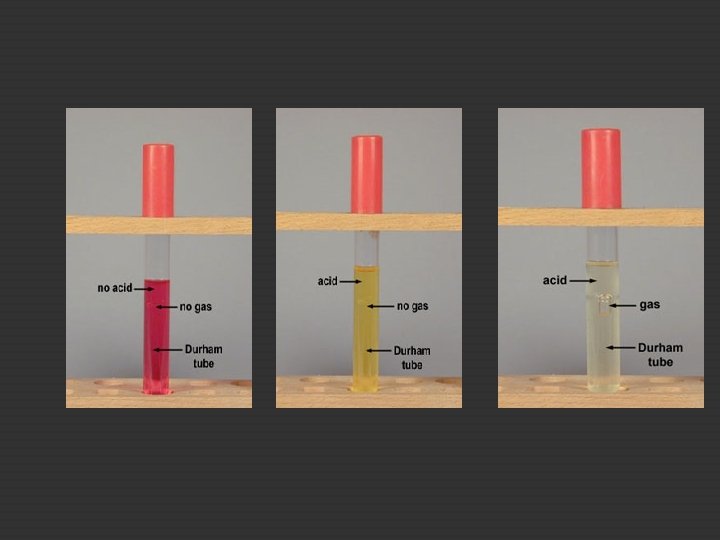
19. A. Fermentation of Carbohydrates A wide variety of carbohydrates may be fermented in order to obtain energy and the types of carbohydrates which are fermented by a specific organism can serve as a diagnostic tool for the identification of that organism. End products of fermentation. • • acid end products. acid and gas end products. Red phenol red in normal p. H, yellow in acid condition
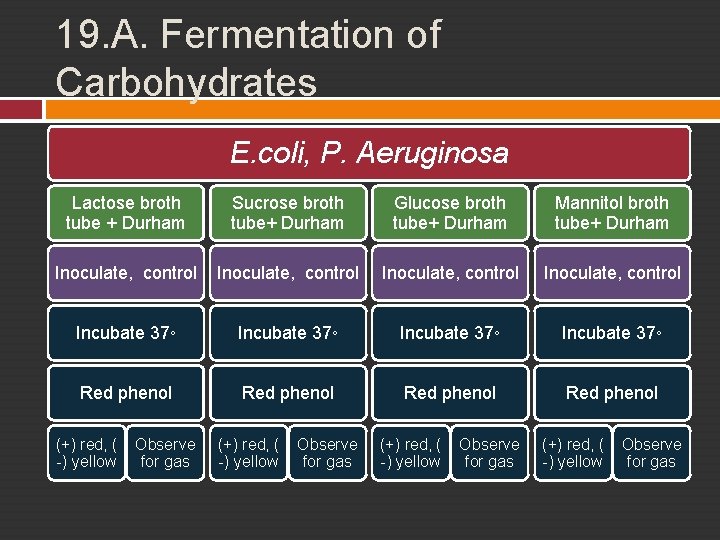
19. A. Fermentation of Carbohydrates E. coli, P. Aeruginosa Lactose broth tube + Durham Sucrose broth tube+ Durham Glucose broth tube+ Durham Mannitol broth tube+ Durham Inoculate, control Incubate 37◦ Red phenol (+) red, ( -) yellow Observe for gas

19. B. Reduction of Nitrate Bacteria can reduce nitrate Anaerobic condition Nitrate reductase enzyme NO 3 - + 2 e- + 2 H- NO 2 - + H 2 O Reagent A Sulfanilic acid + Reagen B alphanaphtylamine, if nitrite is presence (+), red If (-), zinc will reduce nitrate, bring red color, indicates that nitrate did not reduced before, if transparent (+)
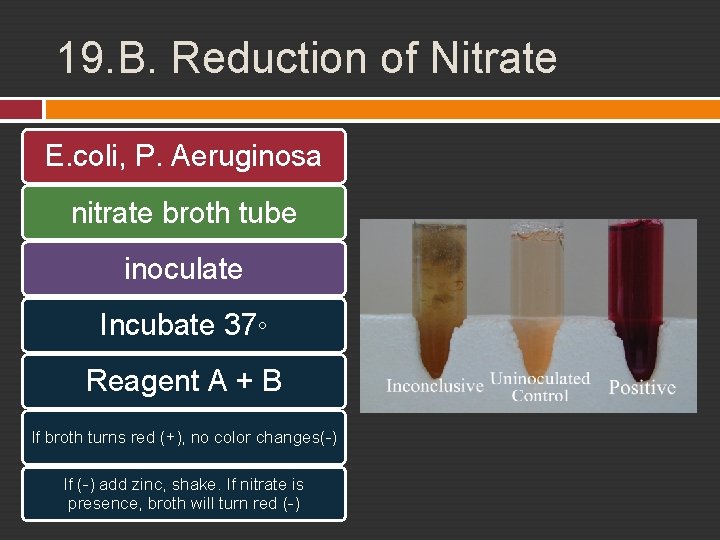
19. B. Reduction of Nitrate E. coli, P. Aeruginosa nitrate broth tube inoculate Incubate 37◦ Reagent A + B If broth turns red (+), no color changes(-) If (-) add zinc, shake. If nitrate is presence, broth will turn red (-)
19. C. Catalase Reaction Aerobic reaction hydrogen peroxide, reactive, destructing enzyme Catalase preventing damage, turning H 2 O 2 into free H 20 and O 2 Superoxide dismutase in species which has no catalase
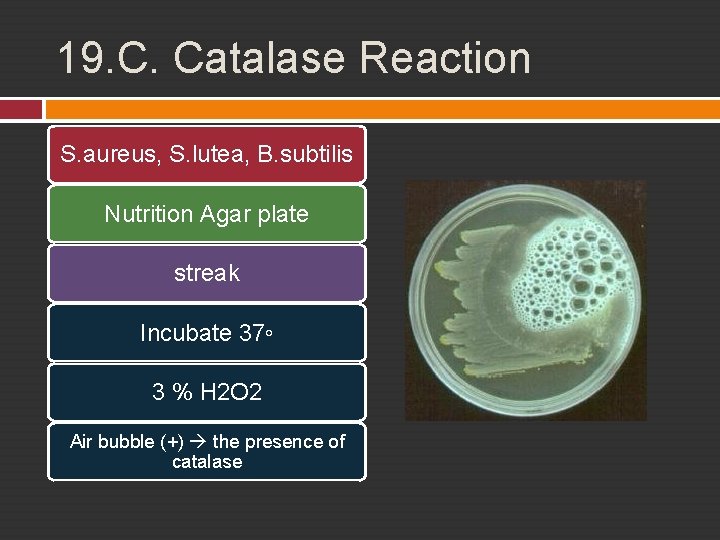
19. C. Catalase Reaction S. aureus, S. lutea, B. subtilis Nutrition Agar plate streak Incubate 37◦ 3 % H 2 O 2 Air bubble (+) the presence of catalase

19. D. Oxidase Test Oxidase enzyme Electron transport system in aerobic resp. p-aminodimethylanyline oxalate artificial substrate, donating electrone and be oxidized into black substances if oxydase and free oxygen are present (+) pink – maroon-black (-) no color change
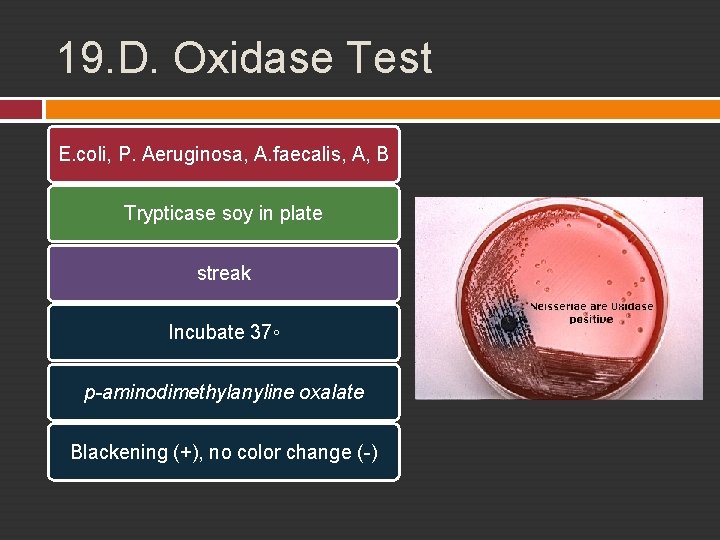
19. D. Oxidase Test E. coli, P. Aeruginosa, A. faecalis, A, B Trypticase soy in plate streak Incubate 37◦ p-aminodimethylanyline oxalate Blackening (+), no color change (-)

Exp. 20 – Acivities of Intracelullar Enzymes : IMVi. C and TSI Test 20. A. 1 Indol Reaction Test 20. A. 2 Methyl Red Reaction 20. A. 3 Vogus-Proskauer Reaction 20. A. 4 Use of Citrate 20. B. Triple Sugar Iron Agar Test
A. IMVic Test Enterobactericeae G. I tract Identification is important in preventing contamination to food and water supply Pathogenic, sometimes pathogenic, normal flora Non Enteric Fermenting lactose Enteric Not fermenting lactose
20. A. 1 Indol Reaction Test Indol =a component of tryptophane, an essential amino acid Will not occur if carbohydrate that needs to be degraded is exsist low p. H Specific characteristic of intestinal bacteria Indol + Kovac reagent red cherry on the surface of the test tube
20. A. 1 Indol Reaction Test E. coli, B. cereus Trypton 1 % broth tube Trypton 1 % + glucose 1 % broth tube streak Streak Incubate 37◦ Kovac reagent Red colors on tube surface (+) Observe for red ring

20. A. 2 Methyl Red Reaction Glucose primary energy source for enteric Some turn glucose into acid (glucose fermentation) low p. H Important in differentiating E. coli and E. aerogenes Methyl red � red : p. H 4 � Yellow : p. H 6
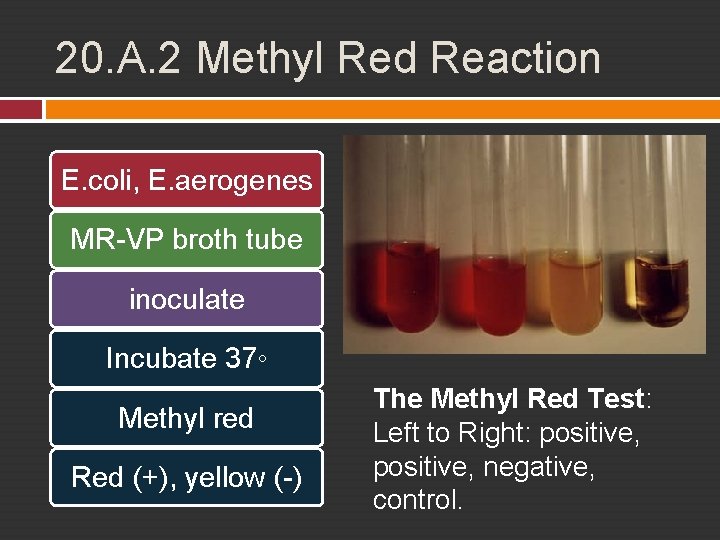
20. A. 2 Methyl Red Reaction E. coli, E. aerogenes MR-VP broth tube inoculate Incubate 37◦ Methyl red Red (+), yellow (-) The Methyl Red Test: Left to Right: positive, negative, control.

20. A. 3 Vogus-Proskauer Reaction Some fermentative organisms do not produce enough stable acids to lower the p. H of the medium. To detect the ability of m. o. In producing non acid substance acetymethylcarbinol Characteristic of E. aerogenes
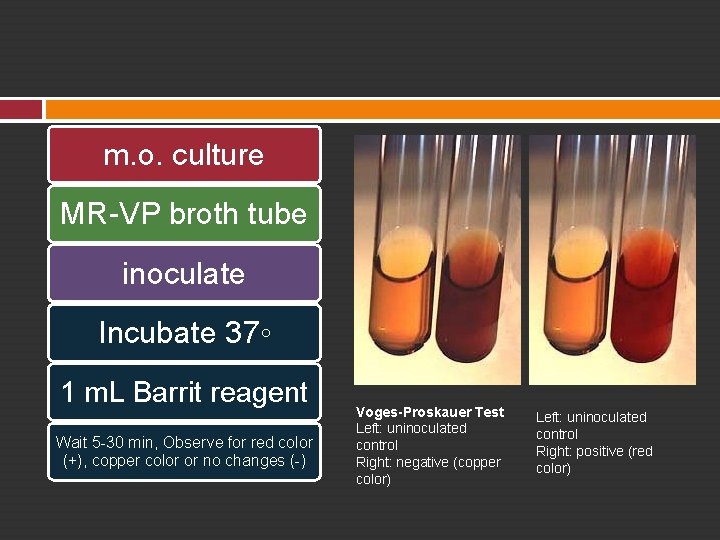
m. o. culture MR-VP broth tube inoculate Incubate 37◦ 1 m. L Barrit reagent Wait 5 -30 min, Observe for red color (+), copper color or no changes (-) Voges-Proskauer Test Left: uninoculated control Right: negative (copper color) Left: uninoculated control Right: positive (red color)

20. A. 4 Use of Citrate When carbohydrate does not present, citrate is used as carbon source in providing energy Citrate permease facilitating citrate transport Citrate oxalatoacetate acid + acetate pyruvic acid + CO 2 during this reaction medium will turn to alkaline condition Bromthymol blue indicator will turn from green at neutral p. H (6. 9) to blue when a p. H higher than 7. 6 is reached (basic or alkaline). If the citrate is utilized, the resulting gowth will produce alkaline products (p. H >7. 6), changing the color of the medium from green to blue.
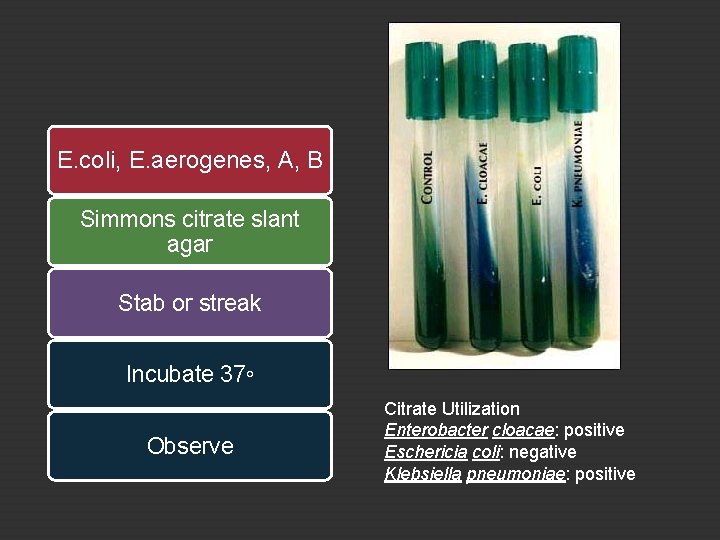
E. coli, E. aerogenes, A, B Simmons citrate slant agar Stab or streak Incubate 37◦ Observe Citrate Utilization Enterobacter cloacae: positive Eschericia coli: negative Klebsiella pneumoniae: positive

20. B. Triple Sugar Iron Agar Test To differentiate Enterobactericeae (bacil, gram -, fermenting glucose, producing acid) vs non Enterobactericeae TSI glucose + lactose + sucrose + phenol red Phenol red as p. H indicator red (alkaline), yellow (acid) � Surface : red, bottom : yellow glucose fermentation only � Surface and bottom : yellow lactose and sucrose fermentation also occur � Surface and bottom : red no carb fermentation
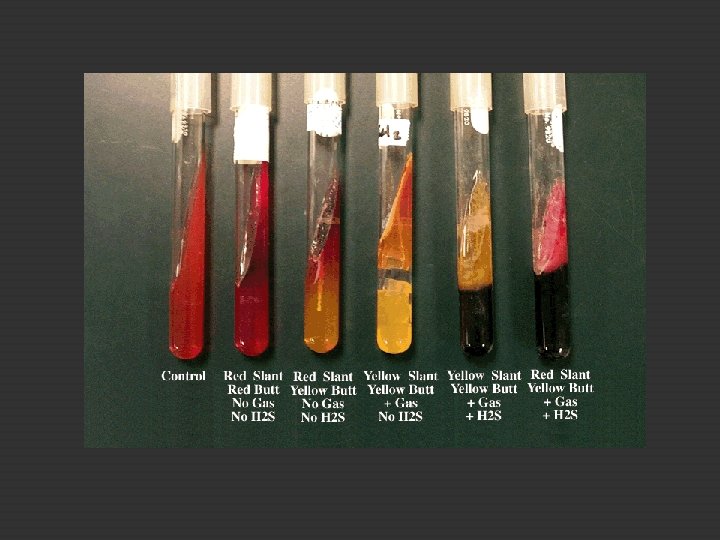

20. B. Triple Sugar Iron Agar Test E. coli, P. Aeruginosa, E. aerogenes TSI slant agar Stab and streak Incubate 37◦ Observe

Exp. 21 – Other Biochemical Activities 21. A. Litmus Milk Reaction 21. B. Urease Test 21. C. H 2 S Production
21. A. Litmus Milk Reaction Several milk substrate reactions using litmus in media: Glucose fermentation litmus act as p. H indicator purple : normal p. H, pink : acid, formation of gas Litmus reduction litmus act as acceptor to bond hydrogen ion purple : oxidized, white : reducted Curd formation acid type (solid) and rennet type (semi solid) Proteolysis forming of ammonia litmus act as p. H indicator Alcaline reaction litmus act as p. H indicator

POSSIBLE REACTION A. Acid/Reduction/Curd B. Reduction/Curd (arrow denotes gas pocket) C. Uninoculated Control D. Acid Formation E. Proteolysis of casein F. Alkaline Reaction

21. A. Litmus Milk Reaction E. coli, P. Aeruginosa, A. faecalis, S. lactis, E. aerogenes Litmus milk broth tube inoculate Incubate 37◦ Observe
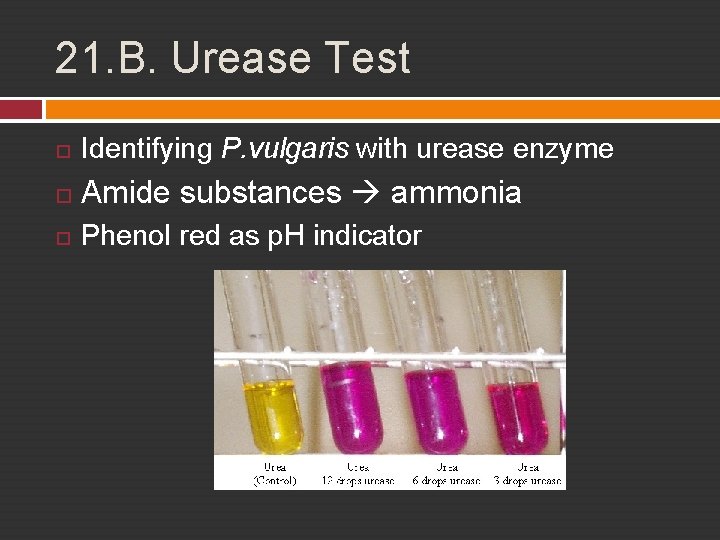
21. B. Urease Test Identifying P. vulgaris with urease enzyme Amide substances ammonia Phenol red as p. H indicator
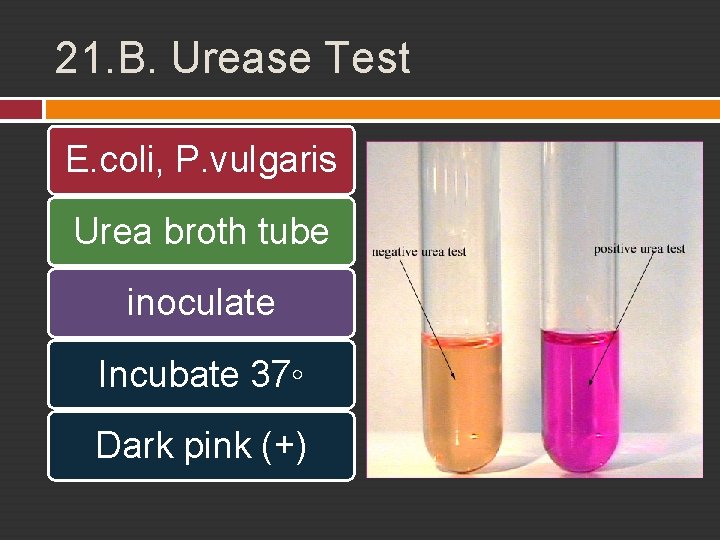
21. B. Urease Test E. coli, P. vulgaris Urea broth tube inoculate Incubate 37◦ Dark pink (+)

21. B. H 2 S Production Some bacteria are capable of breaking down sulfur or reducing inorganic sulfurcontaining compounds to produce hydrogen sulfide (H 2 S). For identifying Proteus and Salmonella. a medium with a sulfur-containing compound and iron salts If the sulfur is reduced and hydrogen sulfide is produced, it will combine with the iron salt to form a visible black ferric sulfide (Fe. S) in the tube.
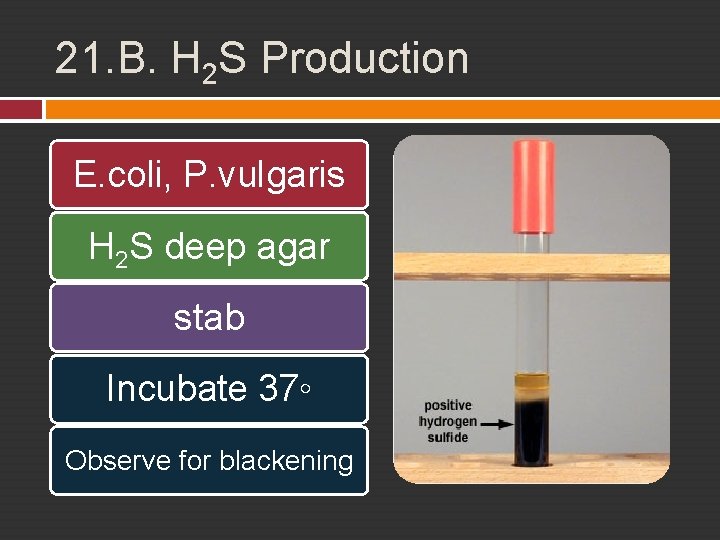
21. B. H 2 S Production E. coli, P. vulgaris H 2 S deep agar stab Incubate 37◦ Observe for blackening

Why all these efforts ? “To identify bacteria, we must rely heavily on biochemical testing. The types of biochemical reactions each organism undergoes act as a "thumbprint" for its identification. ”
- Slides: 64