Environmental Forensics R Paul Philp School of Geology




















- Slides: 56

Environmental Forensics R. Paul Philp, School of Geology and Geophysics, University of Oklahoma, Norman, OK. 73019

Environmental Forensics • What is “Environmental Forensics”? • “Environmental Forensics” can be defined as a scientific methodology developed for identifying petroleum-related and other potentially hazardous environmental contaminants and for determining their sources and time of release. It combines experimental analytical procedures with scientific principles derived from the disciplines of organic geochemistry and hydrogeology. Environmental Forensics provides a valuable tool for obtaining scientifically proven, court admissible evidence in environmental legal disputes. • Much of the information required in this approach will not be obtained from the data obtained using the conventional EPA methods 2

Crude Oils and Related Products

Basic Environmental Forensic Questions • What is the product? • Is there more than one source and, if so, which one caused the problem? • How long has it been there? • Is it degrading?

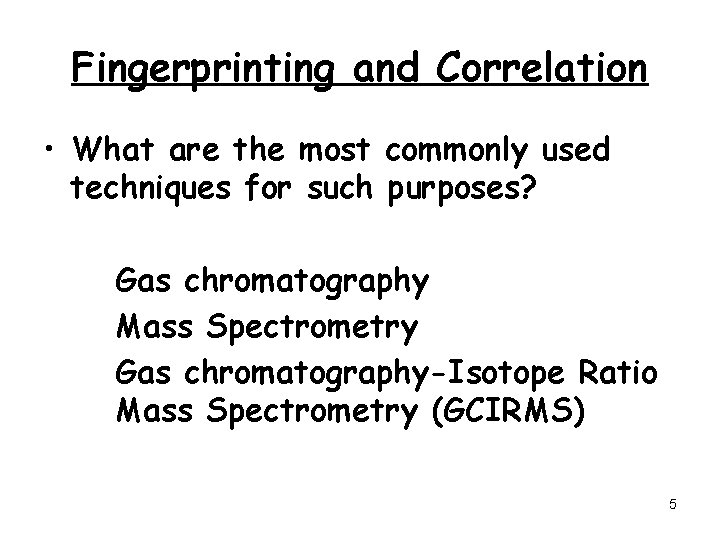
Fingerprinting and Correlation • What are the most commonly used techniques for such purposes? Gas chromatography Mass Spectrometry Gas chromatography-Isotope Ratio Mass Spectrometry (GCIRMS) 5

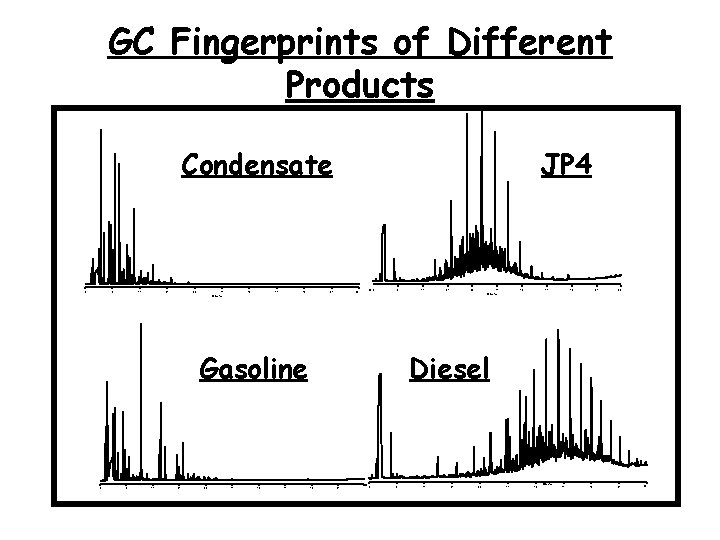
GC Fingerprints of Different Products Condensate 0 7 14 21 28 35 42 49 56 JP 4 63 70 70 0 7 14 21 28 35 Gasoline 0 7 14 21 28 35 42 49 56 63 70 Minutes Diesel 56 63 0 7 14 21 28 35 42 Minutes 49 56 63 70

GC Fingerprints of different products • Although GC permits product identification, many gasoline samples, for example, will be chromatographically similar, even if from different sources. • Refined products generally do not contain biomarkers making GCMS of little consequence. • If refined products are from different sources, stable isotopes may provide a potential solution. Minutes

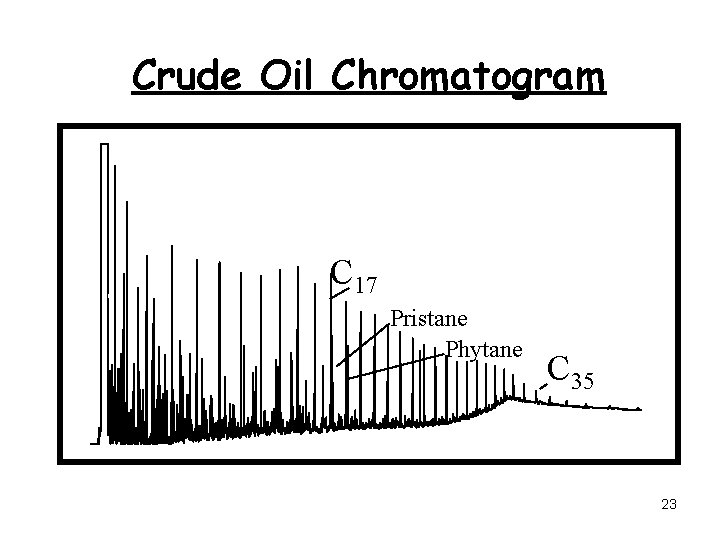
Crude Oil Chromatogram C 17 Pristane Phytane 0 C 35

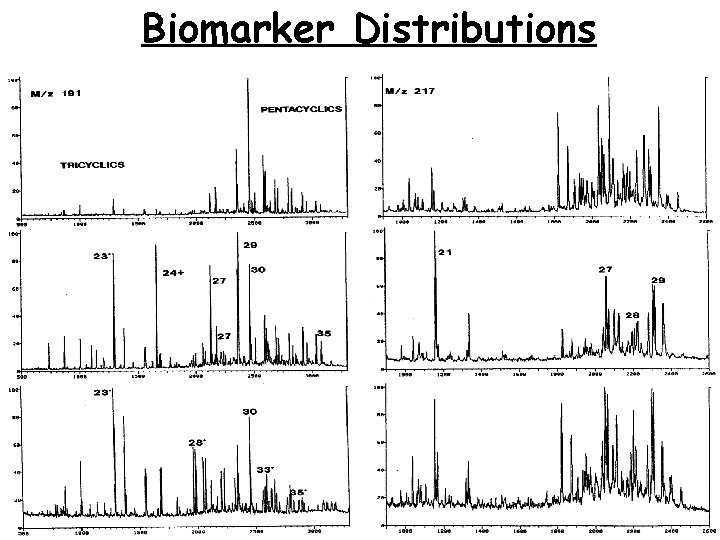
Biomarker Distributions

Utilization of Stable Isotopes • What is the product? NO • Is there more than one source and, if so, which one caused the problem? YES • How long has it been there? NO • Is it degrading? YES

Utilization of Stable Isotopes Why do compounds derived from different feedstocks have different isotope values?

Carbon Isotopes • Carbon in fossil fuels is initially derived from atmospheric CO 2. During photosynthesis, fractionation of the two isotopes occurs with preferential assimilation of the lighter isotopes. 12

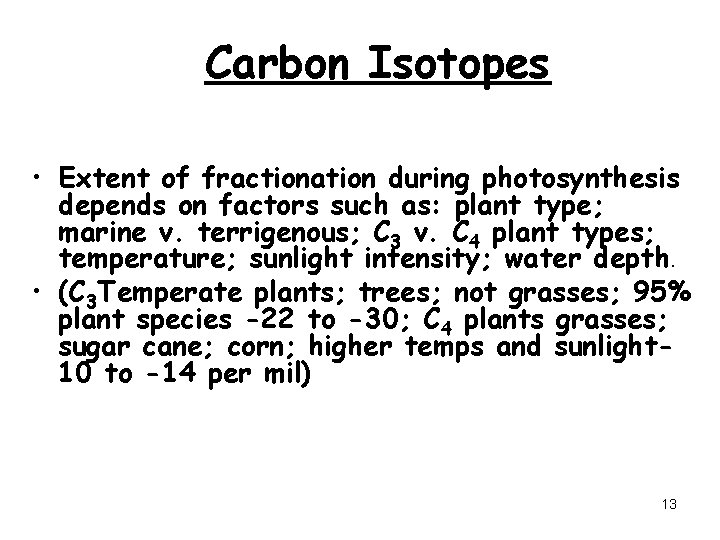
Carbon Isotopes • Extent of fractionation during photosynthesis depends on factors such as: plant type; marine v. terrigenous; C 3 v. C 4 plant types; temperature; sunlight intensity; water depth. • (C 3 Temperate plants; trees; not grasses; 95% plant species -22 to -30; C 4 plants grasses; sugar cane; corn; higher temps and sunlight 10 to -14 per mil) 13

Stable Isotope Determinations ISOTOPIC VALUES CAN BE MEASURED IN TWO WAYS: • BULK ISOTOPES • ISOTOPIC COMPOSITIONS OF INDIVIDUAL COMPOUNDS 14

Isotope Values of Crude Oils Vary with Source d 13 C -35 -30. 06 -30 -29. 52 -29. 68 -29. 50 -25. 66 -25 -23. 45 -23. 27 -20 Monterey Crude Katalla Crude Cook Inlet Crude North Slope Crude Unknown Source Monterey Source NSC Source Petroleum Source 15

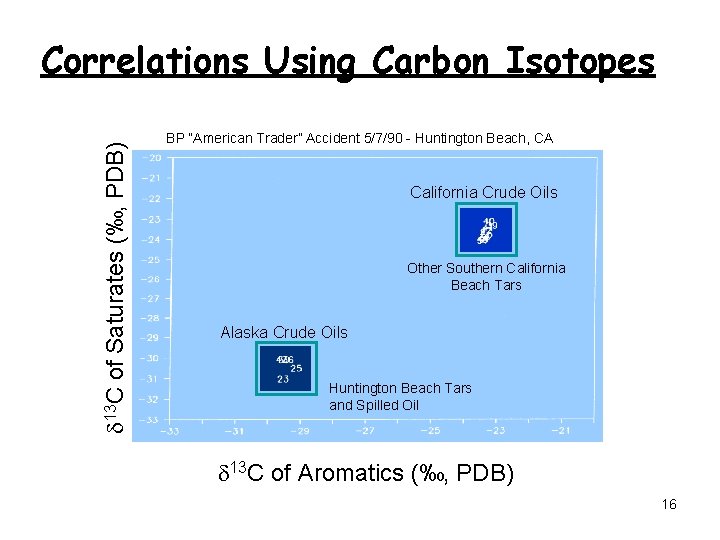
d 13 C of Saturates (‰, PDB) Correlations Using Carbon Isotopes BP “American Trader” Accident 5/7/90 - Huntington Beach, CA California Crude Oils Other Southern California Beach Tars Alaska Crude Oils Huntington Beach Tars and Spilled Oil d 13 C of Aromatics (‰, PDB) 16

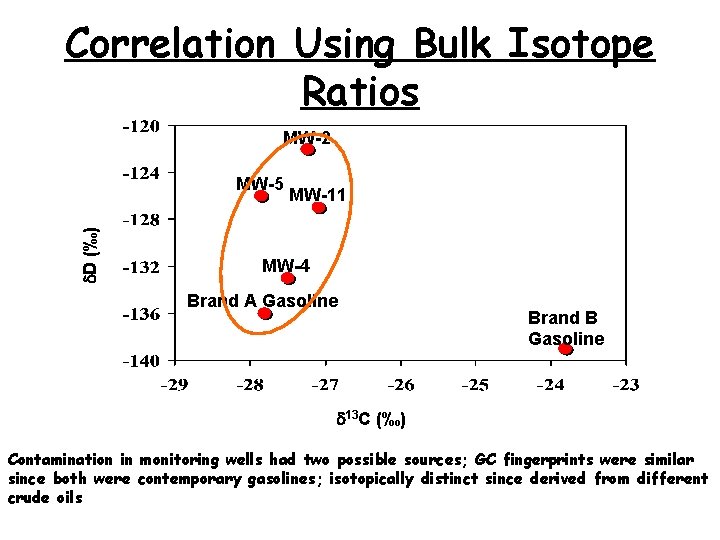
Correlation Using Bulk Isotope Ratios MW-2 d. D (‰) MW-5 MW-11 MW-4 Brand A Gasoline Brand B Gasoline d 13 C (‰) Contamination in monitoring wells had two possible sources; GC fingerprints were similar since both were contemporary gasolines; isotopically distinct since derived from different crude oils

EXXON VALDEZ • March 24 th, 1989 • 258, 000 barrels of Alaskan North Slope crude oil spilled into Prince William Sound

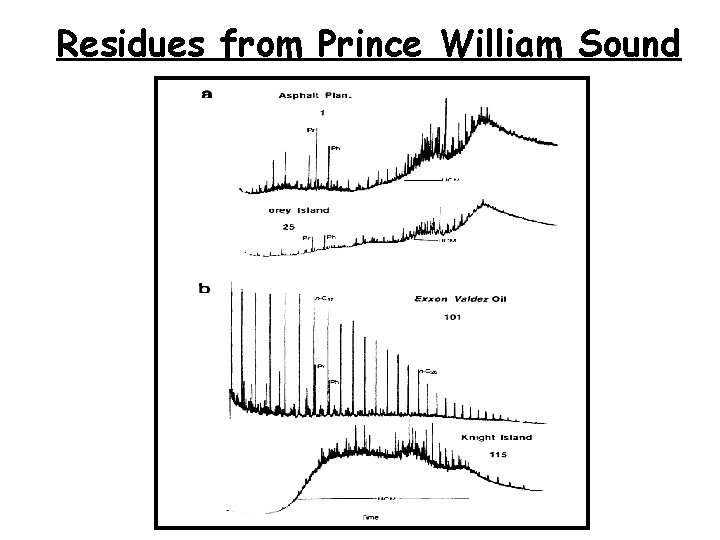
Residues from Prince William Sound

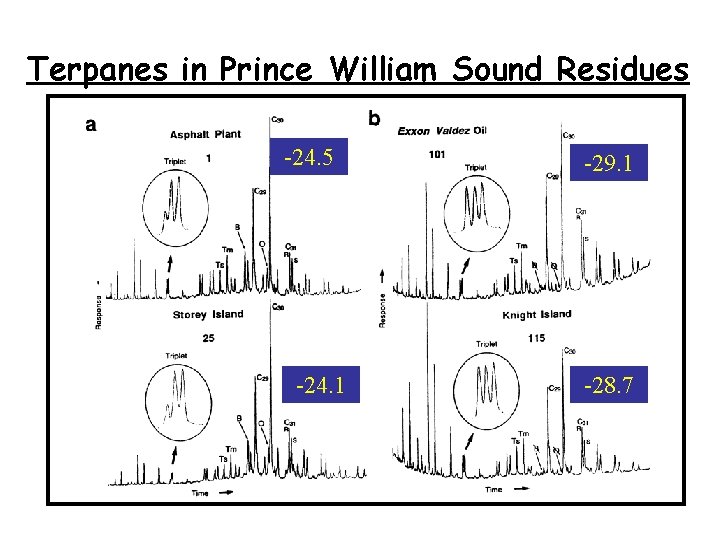
Terpanes in Prince William Sound Residues -24. 5 -24. 1 -29. 1 -28. 7

Stable Isotope Determinations ISOTOPIC VALUES CAN BE MEASURED IN TWO WAYS: • BULK ISOTOPES • ISOTOPIC COMPOSITIONS OF INDIVIDUAL COMPOUNDS 21

GCIRMS System

Crude Oil Chromatogram C 17 Pristane Phytane C 35 0 23

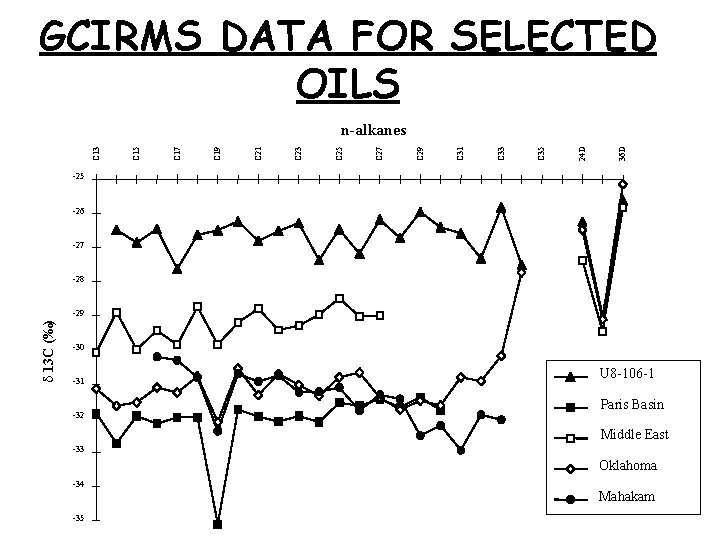
GCIRMS DATA FOR SELECTED OILS 36 D 24 D C 35 C 33 C 31 C 29 C 27 C 25 C 23 C 21 C 19 C 17 C 15 C 13 n-alkanes -25 -26 -27 -28 d 13 C (‰) -29 -30 -31 -32 U 8 -106 -1 Paris Basin Middle East -33 Oklahoma -34 -35 Mahakam 24

Hydrocarbon Spills and Weathering • Major effects of weathering from a geochemical perspective are : – Evaporation – Water washing – Biodegradation 25

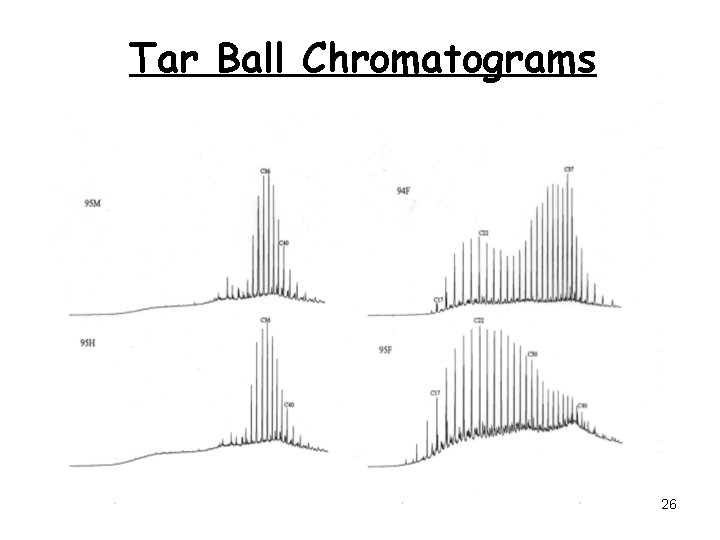
Tar Ball Chromatograms 26

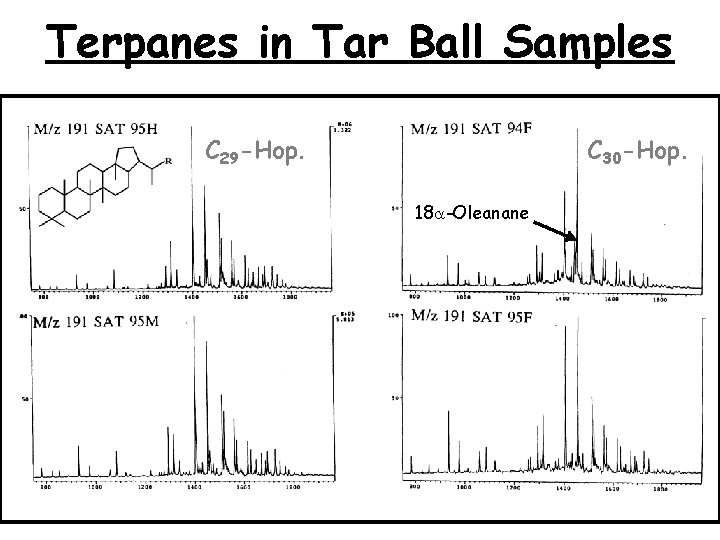
Terpanes in Tar Ball Samples C 29 -Hop. C 30 -Hop. 18 a-Oleanane 27

GCIRMS – Tar Balls 28

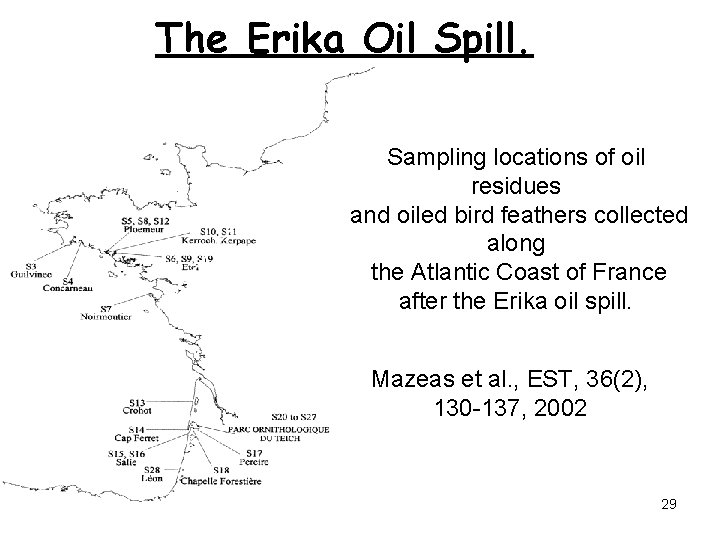
The Erika Oil Spill. Sampling locations of oil residues and oiled bird feathers collected along the Atlantic Coast of France after the Erika oil spill. Mazeas et al. , EST, 36(2), 130 -137, 2002 29

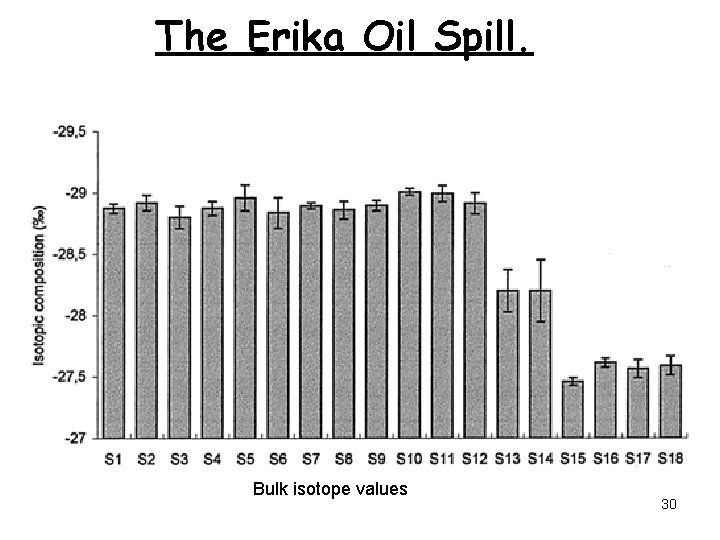
The Erika Oil Spill. Bulk isotope values 30

The Erika Oil Spill. Molecular n-alkane isotopic compositions of the oil residues collected in the north Atlantic shoreline (mean of S 2 -S 12), on the Crohot Beach (S 13), in the Arcachon Bay area (mean of S 14 -S 18), and of bird feathers (mean of S 19 -S 28) are compared with 31 Erika oil.

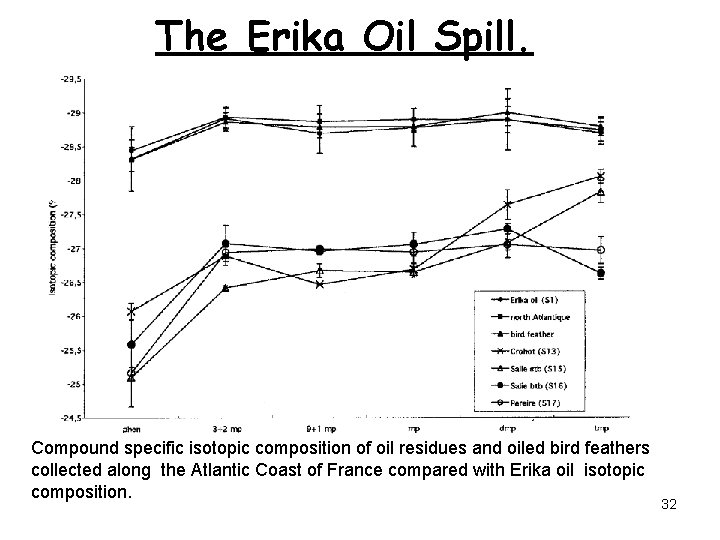
The Erika Oil Spill. Compound specific isotopic composition of oil residues and oiled bird feathers collected along the Atlantic Coast of France compared with Erika oil isotopic composition. 32

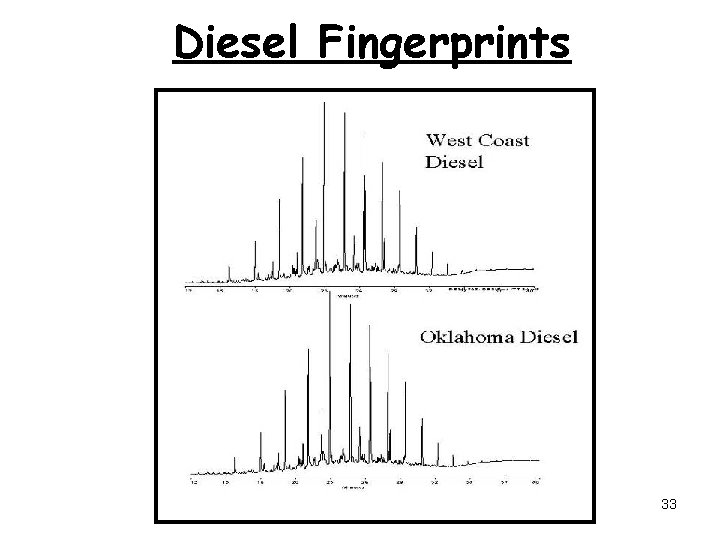
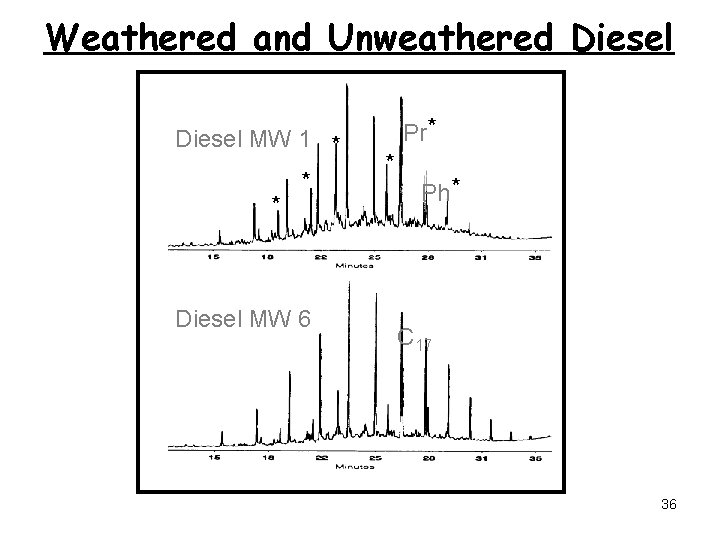
Diesel Fingerprints 33

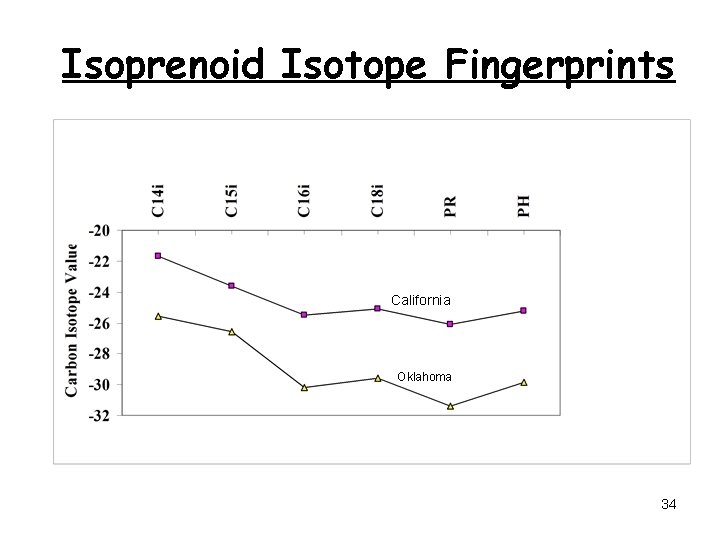
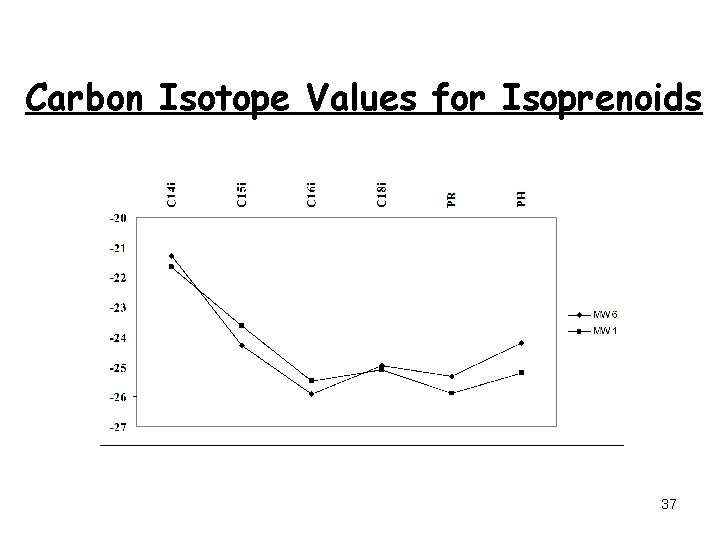
Isoprenoid Isotope Fingerprints California Oklahoma 34

Forensic Geochemistry B Site A MW 1 Groundwater flow direction C MW 6 Site B 35

Weathered and Unweathered Diesel MW 1 * * * Diesel MW 6 Pr* * Ph* C 17 36

Carbon Isotope Values for Isoprenoids 37

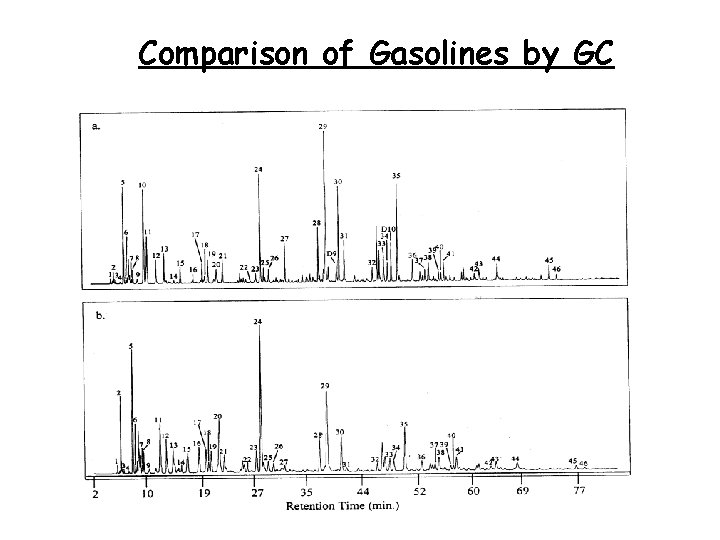
Gasolines • Gasolines from different sources often have very similar chromatograms, making it difficult to distinguish such samples • Gasolines are also devoid of biomarkers, further limiting correlation possibilities • One solution here is to use GCIRMS for both the hydrocarbons and additives

Comparison of Gasolines by GC 39

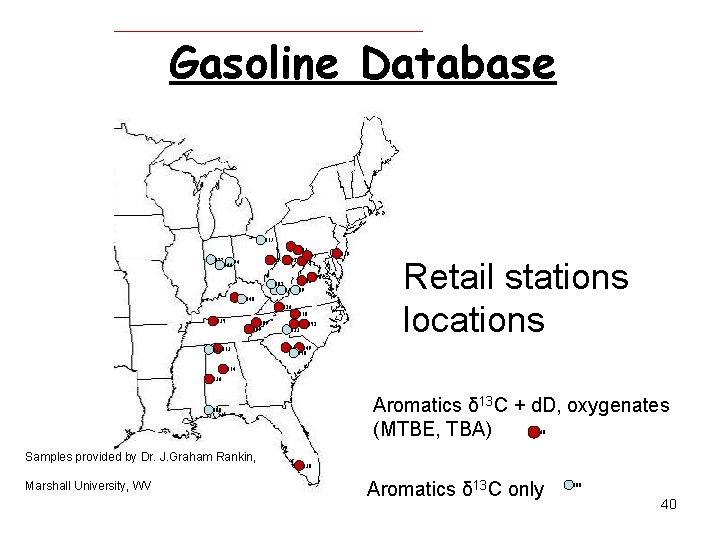
Gasoline Database 117 122 30 83 144 94 31 97 78 52 102 6667 62 90 89 91 148 126 118 135 109 107 133113 151 152 123 119 Retail stations locations 143 149 150 114 136 Aromatics δ 13 C + d. D, oxygenates (MTBE, TBA) 108 138 Samples provided by Dr. J. Graham Rankin, Marshall University, WV 138 Aromatics δ 13 C only 108 40

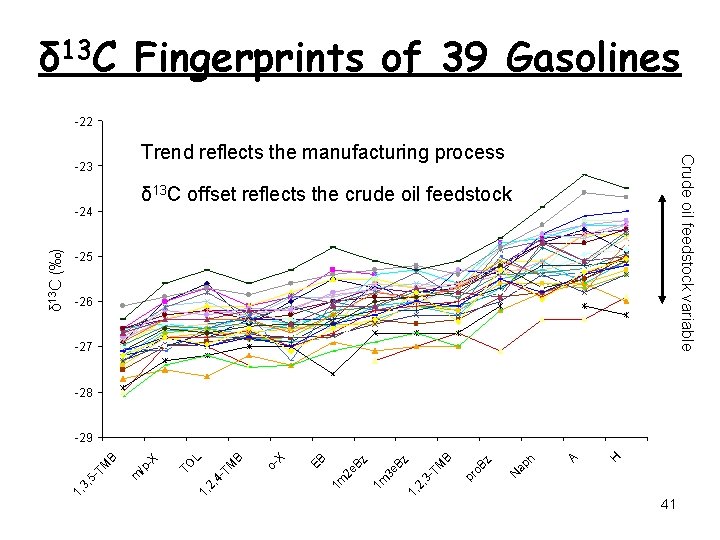
δ 13 C Fingerprints of 39 Gasolines -22 Crude oil feedstock variable Trend reflects the manufacturing process -23 δ 13 C offset reflects the crude oil feedstock -25 -26 -27 -28 H A ap h N z pr o. B B TM 1, 2, 3 - 3 e Bz 1 m 2 e Bz 1 m EB o. X TM B L 1, 2, 4 - TO /p -X m TM B -29 1, 3, 5 - δ 13 C (‰) -24 41

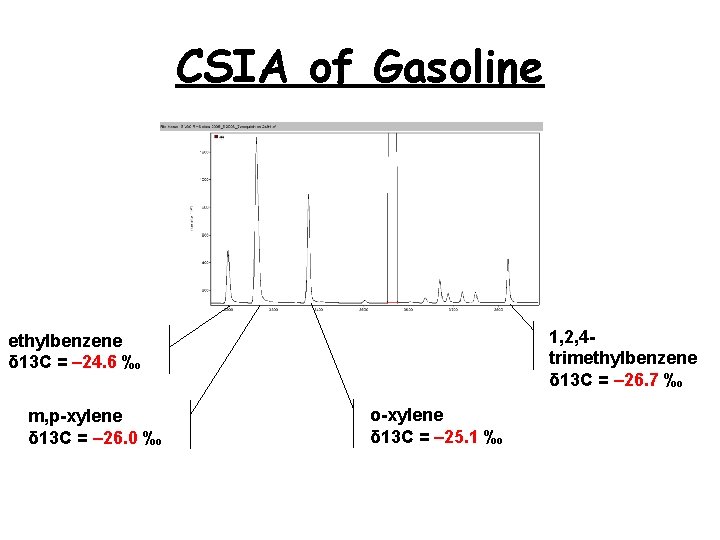
CSIA of Gasoline 1, 2, 4 trimethylbenzene δ 13 C = – 26. 7 ‰ ethylbenzene δ 13 C = – 24. 6 ‰ m, p-xylene δ 13 C = – 26. 0 ‰ o-xylene δ 13 C = – 25. 1 ‰

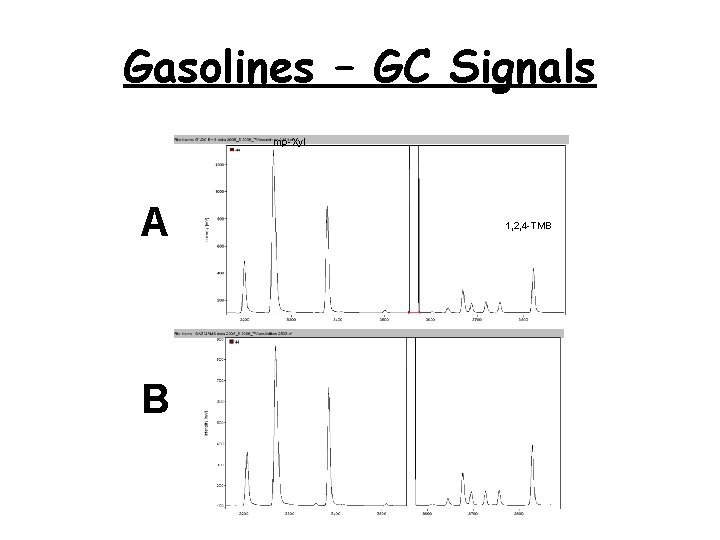
Gasolines – GC Signals mp-Xyl A B 1, 2, 4 -TMB

1, 1, 44 H A ap h N z pr o. B B -T M 3 e Bz 1 m 2, 3 EB o. X 2 e Bz 1 m L B -T M 2, 4 TO /p -X m B -T M 3, 5 δ 13 C (‰) Gasolines – Different δ 13 C Fingerprints -22 A -23 -24 -25 B -26 -27 -28 -29 -30

PCE Degradation Site Study Hunkeler et al. , J. Contaminant Hydrology, 74, 265 -282, 2004.

PCE Source Evaluation Study -33 -27 Hunkeler et al. , J. Contaminant Hydrology, 74, 265 -282, 2004. -25

PCBs • Study by Yanik et al. , OG, 239 -253, 34, 2003 showed that different Aroclors may be isotopically different and thus useful for source discrimination although there is some slight enrichment from degradation. 47

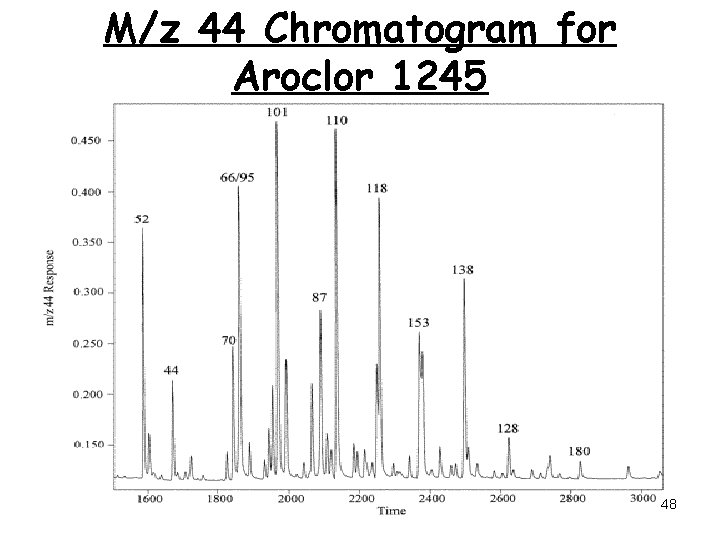
M/z 44 Chromatogram for Aroclor 1245 48

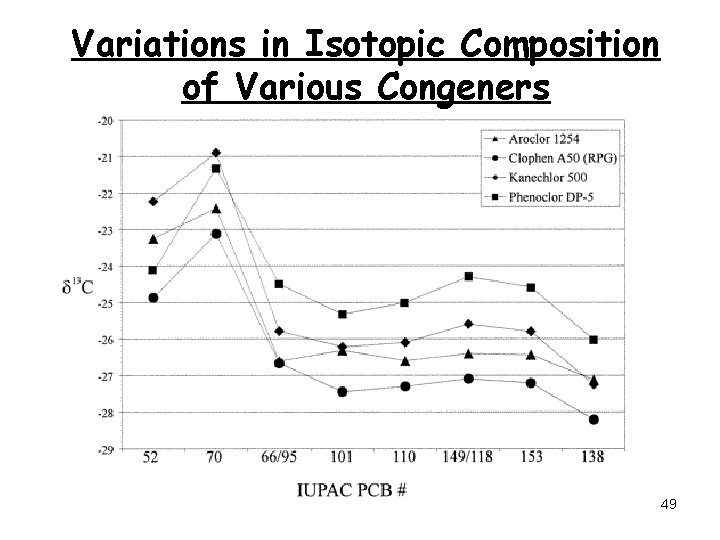
Variations in Isotopic Composition of Various Congeners 49

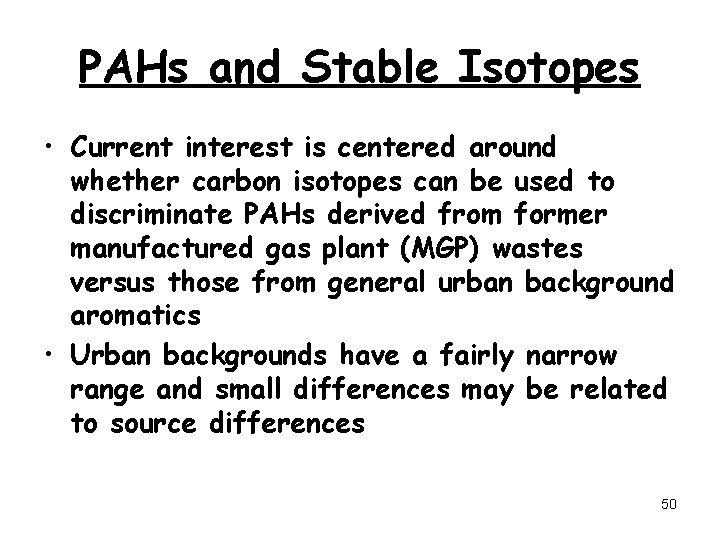
PAHs and Stable Isotopes • Current interest is centered around whether carbon isotopes can be used to discriminate PAHs derived from former manufactured gas plant (MGP) wastes versus those from general urban background aromatics • Urban backgrounds have a fairly narrow range and small differences may be related to source differences 50

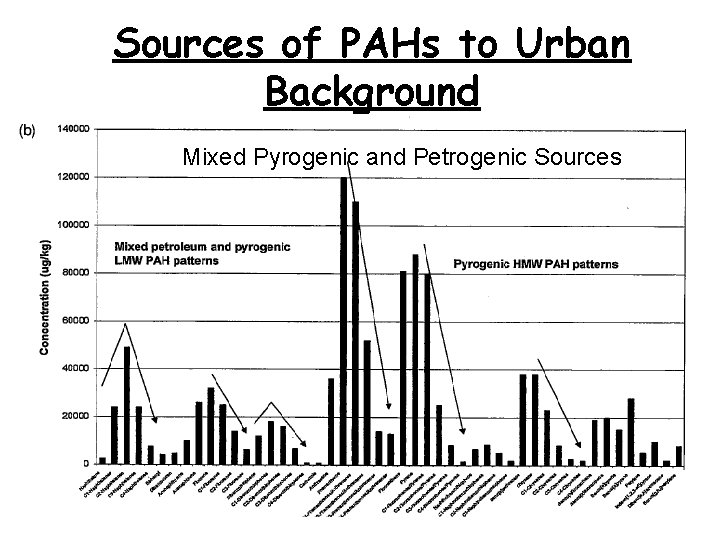
Sources of PAHs to Urban Background Mixed Pyrogenic and Petrogenic Sources 51

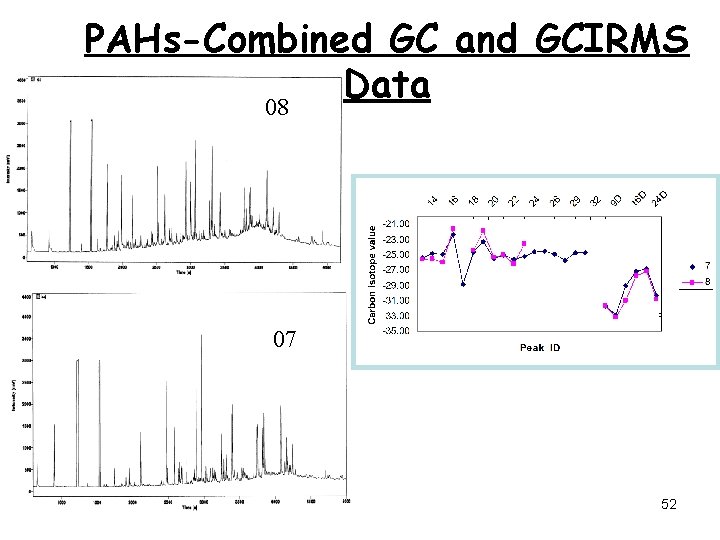
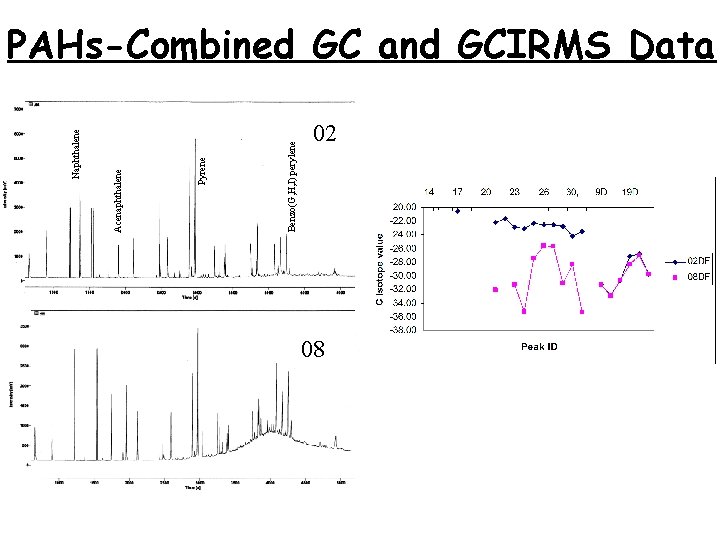
PAHs-Combined GC and GCIRMS Data 08 07 52

Benzo(G, H, I) perylene Pyrene Acenaphthalene Naphthalene PAHs-Combined GC and GCIRMS Data 02 08 08

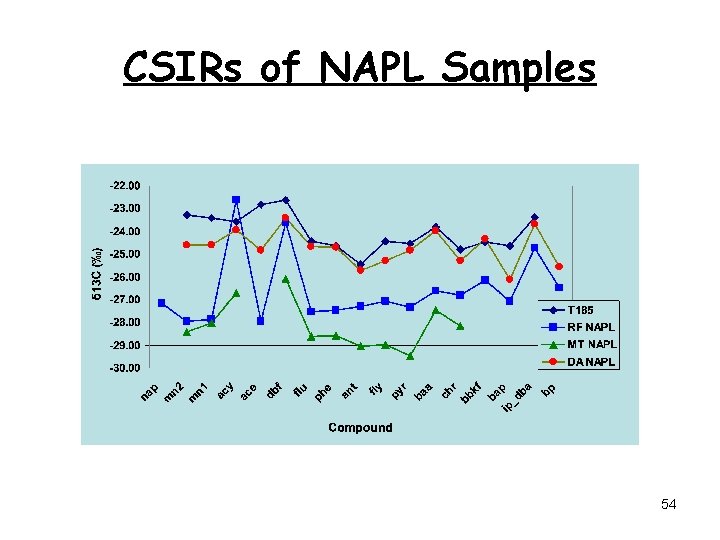
CSIRs of NAPL Samples 54

PAH Fingerprints and Isotopes Show No MGP Contribution to Background MT 03, MT 07, MT 09, MT 10 – background soil samples from town MT NAPL – tar from MGP site in town TPAH-ug/kg Fl/Py MT 03 2, 510 1. 177 MT 07 11, 100 1. 136 MT 09 3, 880 1. 194 MT 10 6, 940 1. 208 MT NAPL 0. 64 55

Summary • Environmental Forensics combines a variety of analytical tools to typically provide information on origin and state of contaminants in the environment. • One of these tools involves utilization of stable isotopes. • In some situations stable isotope data compliments other analytical data. In other cases may be only tool available.
 Richard philp ird
Richard philp ird Hans gross forensic science
Hans gross forensic science Wireless health
Wireless health Geology is the study of
Geology is the study of Geology
Geology What is geology?
What is geology? Structural geology
Structural geology Types of folds geology
Types of folds geology Geology lecture series
Geology lecture series Grain size card
Grain size card Differential stress rock
Differential stress rock Define regolith
Define regolith Mineral council australia
Mineral council australia Arizona state university geology
Arizona state university geology Plunge and trend geology
Plunge and trend geology Geology of cuba:
Geology of cuba: Unconfined aquifer definition
Unconfined aquifer definition Maui geology
Maui geology Thrust geology
Thrust geology Steeply dipping beds
Steeply dipping beds Mica mineral hardness
Mica mineral hardness Gcse geology
Gcse geology Focal point engineering
Focal point engineering Folds
Folds Structural geology
Structural geology Confining pressure mohr circle
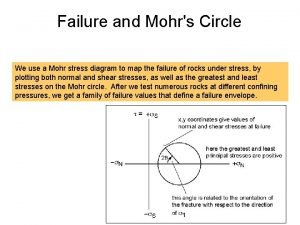
Confining pressure mohr circle Geology earth science definition
Geology earth science definition Symmetrical extinction of minerals
Symmetrical extinction of minerals Astronomy definition earth science
Astronomy definition earth science Earth science vs geology
Earth science vs geology Department of geology university of dhaka
Department of geology university of dhaka Types of folds geology
Types of folds geology Chanel vidal
Chanel vidal Plunging anticline
Plunging anticline Eduqas a level geology
Eduqas a level geology Earth science vs geology
Earth science vs geology Www.geology.sk
Www.geology.sk Geology map of holderness coast
Geology map of holderness coast Define physical geology
Define physical geology Geology vocabulary
Geology vocabulary Stereographic projection structural geology
Stereographic projection structural geology Conformable contact geology
Conformable contact geology Prem model earth
Prem model earth Latvian environment, geology and meteorology centre
Latvian environment, geology and meteorology centre What are sedimenta
What are sedimenta Geology definition
Geology definition Inorganic definition geology
Inorganic definition geology Disconformity definition geology
Disconformity definition geology Geology
Geology Forensic geology definition
Forensic geology definition Rule of v's geology
Rule of v's geology Radioactivity definition geology
Radioactivity definition geology Oil accumulation
Oil accumulation Tectonic joints
Tectonic joints Geology definition
Geology definition Arch
Arch Plant action weathering definition
Plant action weathering definition