Environmental Chemistry Option E 1 1 Pollution Pollution




























































![E 5. 1 - [O 2] • The solubility of O 2(g) in water E 5. 1 - [O 2] • The solubility of O 2(g) in water](https://slidetodoc.com/presentation_image_h/369b2d6c12f7eb777482b94f6496e6de/image-72.jpg)


![E 5. 1 – BOD Values • BOD [pure water] ≈ 1 ppm • E 5. 1 – BOD Values • BOD [pure water] ≈ 1 ppm •](https://slidetodoc.com/presentation_image_h/369b2d6c12f7eb777482b94f6496e6de/image-75.jpg)




![After the algal bloom, [O 2] falls so low that aerobic bacteria cannot survive After the algal bloom, [O 2] falls so low that aerobic bacteria cannot survive](https://slidetodoc.com/presentation_image_h/369b2d6c12f7eb777482b94f6496e6de/image-80.jpg)










































































- Slides: 189

Environmental Chemistry Option

E 1. 1 - Pollution • Pollution refers to changes in the equilibrium (or balance) of biological and non-biological systems, as a result of human activity • Although many so-called pollutants are substances that occur naturally, such as ozone or carbon dioxide, human activity has led to an increase in the concentrations of such substances, which upsets the delicate balance of natural cycles • The atmosphere consists of a relatively thin layer of gas surrounding the Earth. (to 100 km) • By comparison, the earth is 6400 km in radius

E 1. 1 – Layers of the Atmosphere • The atmosphere consists of four layers, separated by a change in temperature gradient • Troposphere -Most of human activity takes place here -Up to 10 -12 km -90% of matter in the atmosphere • Stratosphere • Mesosphere • Thermosphere

E 1. 1 - Troposphere • In the troposphere temperature falls with increasing height, so that at a height of 12 km the temperature is about -55 o. C • This temperature gradient allows convection currents (warm gases rise, cool gases sink) causing mixing in the atmosphere pollutants. • Pollutants at ground level quickly spread throughout the troposphere (not true in stratosphere). • Also, horizontal movement of air masses (wind) causes lateral dispersion of pollutants as well.

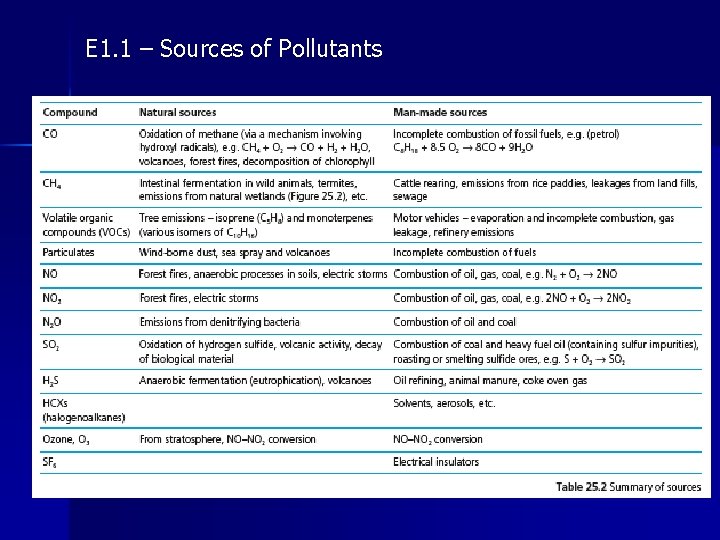
E 1. 1 – Atmospheric Pollutants • As discussed, many gases we consider pollutants, also occur naturally (CO, CO 2, etc) • Anthropogenic, or man-made, output is often not even a large proportion of the worldwide total but can cause issues due to high concentrations in localized areas. • Many atmospheric pollutants arise from the combustion of fossil fuels in motor vehicles or in power stations. This effect has the potential to be minimized

E 1. 1 – Sources of Pollutants

E 1. 2 – Reduction of Pollution • In the internal combustion engine, hydrocarbon fuels are mixed with air, injected into a cylinder and ignited with a spark • The resultant explosion forces the piston to move and is converted to the rotation of the crankshaft, which in turn drives the wheels of the vehicle • We can have complete, partial, and incomplete combustion of the hydrocarbon, ideally it would be complete as follows: 2 C 8 H 18 + 25 O 2 → 16 CO 2 + 18 H 2 O � Ratio of air/fuel (mass) is approx 15: 1

E 1. 2 – Incomplete Combustion • When the ratio of air/fuel (mass) is less than 15: 1, the mixture is said to be ‘rich’ and incomplete combustion ensues • This results in the formation of carbon monoxide C 8 H 18 + 10 O 2 → 3 CO 2 + 5 CO + 9 H 2 O • These unburnt hydrocarbon molecules (CO) are called volatile organic compounds (VOC’s) from exhaust • A very poorly designed (or maintained) engine may emit solid particles (soot) from the exhaust (rich) C 3 H 8 + 2 O 2 → 3 C + 4 H 2 O

E 1. 2 – Reaction with Nitrogen • When the air/fuel ratio is increased the mixture is said to be ‘lean’. A lean mixture will not produce carbon monoxide • When a mixture becomes ‘lean’ (excess O 2) the likelihood that oxygen will react with nitrogen in the air (78%) increases. • Under extreme conditions (like in an engine) the two elements can combine to form nitrogen oxides NOx which leads to ‘misfire’ in the engine (known as knocking) because the fuel ignites before the spark

E 1. 2 – Rich vs Lean • An engine that runs ‘rich’ will produce a lot of power, but with poor fuel consumption and high emissions of CO and VOC’s • An engine that runs ‘lean’ will produce less power, less CO and VOC’s, consume less fuel, but may produce more nitrogen oxides. When mixture is too lean, misfiring causes rise in VOC production N 2 + O 2 → 2 NO • (remember the radical? ) 2 NO • + O 2 → 2 NO 2 �Localized in urban areas, causing health effects and contribute to the formation of acid rain

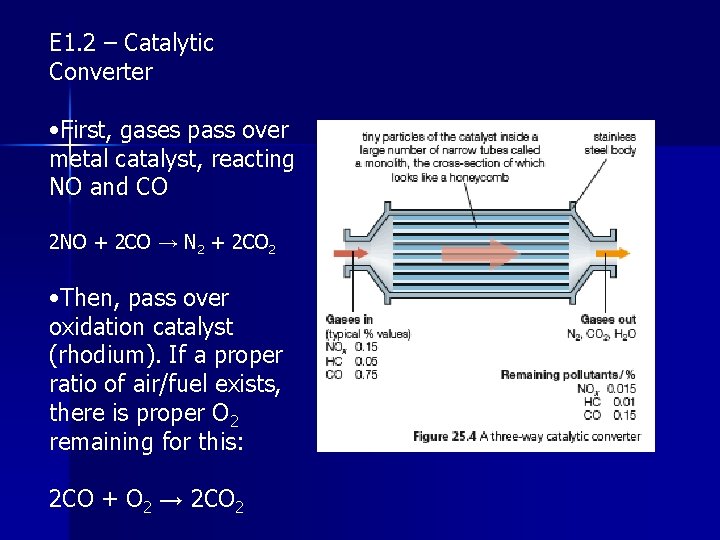
E 1. 2 – Catalytic Converter • Three way catalytic converter is placed in exhaust systems of cars to treat the exhaust gases • Consists of a fine mesh or honeycomb of ceramic material or metal, coated with a thin layer of finely divided platinum, rhodium, or palladium (these T-metals act as heterogeneous catalysts) • Three way refers to: 1. Reduction of nitrogen monoxide to nitrogen 2. Oxidation of unburnt hydrocarbons to carbon dioxide and water vapor 3. Oxidation of carbon monoxide to carbon dioxide

E 1. 2 – Catalytic Converter • First, gases pass over metal catalyst, reacting NO and CO 2 NO + 2 CO → N 2 + 2 CO 2 • Then, pass over oxidation catalyst (rhodium). If a proper ratio of air/fuel exists, there is proper O 2 remaining for this: 2 CO + O 2 → 2 CO 2

E 1. 2 – Sulfur as Pollutant • SO 2 (sulfur dioxide) is an important primary pollutant. - It’s a pungent smelling toxic gas - damages the respiratory system and may lead to asthma attacks. - Highly soluble in water - Contributes to formation of acid rain

E 1. 2 – Primary/Secondary Pollutants • Primary pollutants are emitted directly from the sources and remain unchanged once they enter the environment (particulate matter, inorganic gases, SO 2, etc) • Secondary pollutants are formed in the atmosphere by chemical reactions involving primary pollutants and gases normally present in the air. • Most man-made sulfur dioxide emissions arise from the sulfur that exists as an impurity in coal which is burned extensively in many power plants

E 1. 2 – Sulfur reactions • Sulfur is oxidized during the combustion process S + O 2 → SO 2 • Sulfur dioxide dissolves and reacts with water to produce sulfurous acid, H 2 SO 3 SO 2 + H 2 O → H 2 SO 3 • Sulfur dioxide also undergoes photochemical oxidation in the atmosphere. This occurs in water droplets in which SO 2 is dissolved, and is catalyzed by particulates (soot, etc) 2 SO 2 + O 2 → 2 SO 3 • Sulfur trioxide dissolves and reacts with water to produce sulfuric acid SO 3 + H 2 O → H 2 SO 4

E 1. 2 – Reducing SO 2 Emissions • There are three methods by which sulfur dioxide emissions from power stations can be limited �The coal or oil can be refined to remove sulfur before combustion �Fluidized bed combustion (FBC) reduces the amount of sulfur oxides resulting from combustion �Flue gas desulfurization (FGD) removes sulfur dioxide from the exhaust gases before they leave the power station flue (chimney)

E 1. 2 – Fluidized Bed Combustion • This process suspends the solid coal on an upward flowing jet of air during combustion • Coal dust is mixed with limestone powder (Ca. CO 3) and blasted into the furnace with a jet of air. The jet of air suspends the solid particle so they flow like a fluid Ca. CO 3 → Ca. O + CO 2 2 Ca. O + 2 SO 2 + O 2 → 2 Ca. SO 4 • The Calcium sulfate can then be removed by electrostatic precipitation

E 1. 2 – Flue Gas Desulfurization • Sulfur dioxide emissions can be removed from the flue gases by passing the gases through a suspension of calcium carbonate and calcium oxide in water. Product is calcium sulfite Ca. CO 3 + SO 2 → Ca. SO 3 + CO 2 Ca. O + SO 2 → Ca. SO 3 • Calcium sulfite is then further oxidized, producing calcium sulfate 2 Ca. SO 3 + O 2 → 2 Ca. SO 4

E 1. 2 – Particulate Emissions • Particulate emission refers to the generation of small particles of solid or liquid. Some such pollutants are visible to the naked eye, but most are too small to be seen. �Metal particles �Metal oxide particles �Fly ash �Asbestos dust �Organic particles �Aerosol mist

E 1. 2 – Fly Ash • Fly ash is a combination of very fine carbon, hydrocarbon and metal oxide particles released during the combustion of fossil fuels • Most fly ash is filtered out in flues, but some very fine particles escape into the atmosphere

E 1. 2 – Removal of Particulates • Via sedimentation which relies on letting heavy particles settle out under gravity, or by filtration, in which simple fabric filters capture particles. • Most effective method is electrostatic precipitation – which has two sections �Ionization section, consists of a mesh with thin wires, carrying an electrical charge. Flue gas passes through this mesh, causing any solid or liquid particles to acquire charge �Collection section, consists of metal plates carrying the opposite charge. Particulates are attracted to the plates, and stick. Plates are shaken at intervals to dislodge the build-up layer of particles, then they are removed.


Enviro Chemsitry Part 2 – Acid Deposition

Acid Deposition refers to the process by which acidic particles leave the atmosphere. The most well known example is acid rain • but acidic substances may also be removed by snow and fog, as well as by dry processes involving gases and solid particles. • Production of SO 2 (as discussed in Part 1) aids in this process

E 2. 1 – Carbonic Acid • Natural rain water is acidic, with a p. H around 5. 6 • The acidity of rain is a result of CO 2 naturally present in the atmosphere • When CO 2 is dissolved in water it’s referred to as carbonic acid (H 2 CO 3) but only a very small amount actually exists as a solution H 2 O + CO 2 → H 2 CO 3 • Carbonic acid molecules immediately dissociate in water to form hydrogencarbonate ions, HCO 3 -, and hydronium ions, H 3 O+ H 2 CO 3 + H 2 O → HCO 3 - + H 3 O+

E 2. 1 – Wet Deposition • The most important sources of acid rain are the sulfur oxides produced in power stations • When sulfur oxides dissolve and react in rain water, solutions of sulfuric acids are formed (as discussed in E. 1) SO 2 + H 2 O → H 2 SO 3 + H 2 O → H 2 SO 4 �Another route to sulfuric acid is a gas-phase reaction of a sulfur dioxide molecule with a hydroxyl radical, OH • , to give sulfuric acid SO 2 + OH • → HSO 3 • + • OH → H 2 SO 4

E 2. 1 – Wet Deposition • Nitrogen Oxides also contribute to acid rain. Formed in vehicle engines: HO • + NO • + M → HNO 2 + M �M represents the ‘third body’ which is an inert molecule which absorbs some of the excess energy of the reaction (in the atmosphere M is generally N 2). • NO 2 is formed by the oxidation of NO in the atmosphere and reacts with HO • + • NO 2 + M → HNO 3 + M

E 2. 1 – Wet Deposition • These acids may be deposited in places other than water, such as snow and fog. • Fog is a particular problem for high-altitude forests • The lower temperature at high altitudes causes water vapor to condense out of the atmosphere, forming a moist ‘blanket’ of acidic fog which surrounds trees.

E 2. 1 – Dry Deposition • Dry deposition refers to acidic substances such as gases and particulates leaving the atmosphere in the absence of precipitation (without rain or fog) • Heavy particulate particles may settle out of the atmosphere under gravity. • Acidic gases such as sulfur dioxide may have directly harmful effects on the environment without first being dissolved in rain water.

E 2. 2 – Environmental Effects • Acid deposition effects the environment in 5 ways 1. It affects the p. H of lakes/rivers, which impacts organisms living there 2. It affects the availability of metal ions in soil, which goes on to affect nearby plant life and surface water 3. It directly affects plants 4. It affects buildings and other materials 5. It directly affects human health

E 2. 2 – Impact(1): Lakes/Rivers • Below a p. H of 5. 5 -Some species of fish (salmon) are killed -Algae, zooplankton, which are food for larger organisms -Prevents hatching of fish eggs • Fish are also killed when aluminum, leached from the soil by acid rain, enters lakes and rivers. • The function of fish gills is affected by Al, leaving the fish unable to extract oxygen from the water

E 2. 2 – Impact(2): Soil • The p. H of soil is a key factor which species of plants will grow • Aluminum (naturally present in soil) forms insoluble hydroxide (Al(OH 3) at high p. H values. • When p. H falls due to acid rain, Al becomes soluble and is released into soil. • Other ions (Mg, Ca, etc) which are essential for plant growth are washed away in the same fashion. 2 Al(OH)3 + 2 H 2 SO 4 → Al 2(SO 4)3 + 3 H 2 O

E 2. 2 – Impact(3): Plants • Beyond damaging soil, and lowering available nutrients, acid rain can also damage plants directly • Acid deposition can damage leaf chlorophyll, turning leaves brown and reducing the photosynthetic ability of the plant

E 2. 2 – Impact(4): Buildings • Limestone and marble are forms of Ca. CO 3 which can be eroded by acid rain: Ca. CO 3 + H 2 SO 4 → Ca. SO 4 + H 2 O + CO 2 • Metallic structures (mainly steel, Fe, Al) are readily attacked. The sulfur dioxide gas may attack directly as follows: Fe + SO 2 + O 2 → Fe. SO 4 • Sulfuric acid may attack Fe as well: Fe + H 2 SO 4 → Fe. SO 4 + H 2 Fe + 2 H+ → Fe 2+ + H 2

E 2. 2 – Counteract Acid Dep. • Limit (lower) the amount of acidic substances released to the atmosphere �NOx are removed from vehicle emissions with a catalytic converter �SO 2 emissions from coal power plants can be decreased in several ways (scrubbers, etc) • Addition of compounds that will aid in neutralizing acidic effects �Addition of limestone (Ca. CO 3) �Addition of calcium hydroxide (Ca(OH)2)

Topic E – Enviro Chemistry Part 3 – Greenhouse Effect

E 3. 1 – Greenhouse Effect • Greenhouse gases allow the passage of incoming solar short-wavelength radiation but absorb the longer-wavelength radiation from the Earth. Some of the absorbed radiation is reradiated back to Earth. • TOK: Some people question the reality of climate change and question the motives of scientists who have “exaggerated” the problem. How do we assess the evidence collected and the models used to predict the impact of human activities?

• The greenhouse effect is the cause of the phenomenon of global warming in which the average temperature of Earth rises, causing various environmental disasters • The greenhouse effect itself is absolutely necessary for the Earth to regulate its temperature at a habitable level. • Humans are thought to impact this delicate balance by disrupting the natural equilibrium in the atmosphere, causing the planet to become warmer

Earths “Average” Temp. • Average temperature in the troposphere 14 -15 o. C • Maintained bc the energy incident on Earth (from sun), is balanced by the energy leaving Earth (to space) • Most radiation from the sun is in the visible region, also along with “near UV” and near “IR radiation” • Only 47% of energy directed at the earth is absorbed, remainder is reflected back to space • The peak radiation is 500 nm and is not absorbed by atmospheric gases so is absorbed and radiated by the earth to the rest of the atmosphere

• When Earth absorbs energy, surface temperature rises, and energy flows from hot (earth) to atmosphere (cold), etc • Once energy is re-radiated from the earth, it’s no longer in the visible region, it’s wavelength is much longer and in the infrared region • If all this energy released into space instead, our average atmospheric temp would be -20 o. C • Gases such as H 2 O and CO 2 help to re-radiate energy in the atmosphere and toward the earth as well. It can be seen that an increase in [IR absorbing gases] such as CO 2 and H 2 O results in a decreased amount of energy escaping from the Earth by moving toward the surface

E 3. 2 – Factors for GH Gases • A contribution of a greenhouse gas to the warming of the atmosphere depends on three factors: 1. The abundance of the gas in the atmosphere 2. The ability of the gas to absorb infrared radiation 3. The lifetime of the gas molecules in the atmosphere, before being removed by chemical processes The 2 nd and 3 rd factors are often combined to give a figure called the Global Warming Potential (GWP)

E 3. 2 – Greenhouse Gases • Major Contributors as Greenhouse Gases �Water vapor, H 2 O �Carbon dioxide, CO 2 �Methane, CH 4 �Nitrous Oxide, N 2 O �Chlorofluorocarbons, CFC’s �Ozone, O 3

GH Gases – H 2 O • Most important GH Gas, has a GWP of 0. 1 • Percentage of H 2 O(g) in atmosphere 1 -4%, ranges • Absorbs IR over a broad range of frequencies • Increased atmospheric temperatures lead to more rapid evaporation of the oceans, and larger capacity of the air to carry water vapor (humidity) • Estimates of H 2 O’s contribution to Global Warming is 36%-75%

GH Gases – CO 2 • Percentage of CO 2 in atmosphere is 0. 035% • CO 2 has a GWP of 1. • More efficient than water in absorbing IR radiation • Absorbs IR in a “window” that H 2 O does not • [CO 2] rise due to the following human activities: • Combustion of fossil fuels • Manufacture of cement (Ca. CO 3 → Ca. O + CO 2) • Deforestation in tropics, lower rate of photosynthesis, meaning CO 2 is entering atmosphere more quickly than removed

GH Gases – CH 4 • Percentage in atmosphere CH 4 = 1. 7 x 10 -4 • It’s GWP is 25 (compare to H 2 O=0. 1 and CO 2=1) • Estimates say 4%-9% contribution to Global Warmíng • It is removed from the atmosphere relatively quickly • Formed when cellulose (plant fiber) decomposes anaerobically via bacteria (CH 2 O)n → CH 4 + CO 2 • Occurs on large scale as a result of human actions: - Rice cultivation (paddy fields) - Fermentation of grass in cows, and rotting manure - Leaking gas pipelines - Fermentation of organic materials in covered landfills

GH Gases – N 2 O • GWP of 296. It’s less efficient at absorbing IR than CO 2 but it’s high number comes from a long residence in the atmosphere • Percentage in atmosphere 0. 031% • Accounts for 5% of Global Warming effects • Human activity only accounts for 10 -12% of it’s production, but anthropogenic NO 2 from: - Industrialized agriculture, N fertilizers - Industrialized livestock farming, poor handling of animal waste - Chemical industry, HNO 3 and nylon production

GH Gases – CFC’s • Chlorofluorocarbons (CFC’s) have largely been replaced in aerosols, propellants, and refrigerants by hydrochlorofluorocarbons (HCFC’s) and hydrofluorocarbons (HFC’s) • These gases are less damaging to the ozone layer but still have GWP values much higher that CO 2 and are important contributors to global warming

E 3. 3 – Influence of Greenhouse Gases Rising Sea Levels • As atmospheric temperature increases, sea levels will rise for two reasons: • The increased atmospheric temperature causes accelerated melting • This does not include floating ice in the arctic as it already displaces water while it floats • As oceans warm up, the water in them will expand, occupying more volume (even minor amounts could be significant due to the quantity of water in the ocean!)

Glacier Retreat • Glaciers undergo a seasonal melting and freezing as temperatures vary throughout the year. • In the Himalayas glacial melt water is an important source of fresh water, feeing the rivers of South Asia • Increased melting increases erosion and risk of flooding downriver, a particular problem in low-lying countries

Changing Patterns of Agriculture • In temperate regions (such as Europe) yields of grain will most likely increase due to higher temperature, longer growing season and increased [CO 2] available for photosynthesis • But, increased humidity and rainfall could lead to increased incidence of fungal crop diseases, and migration of tropical insects to higher altitudes. • At higher latitudes, more workable land may become available due to thawing and temperature changes. • Worldwide, the possibility of extreme weather increases the likelihood of ruined harvest

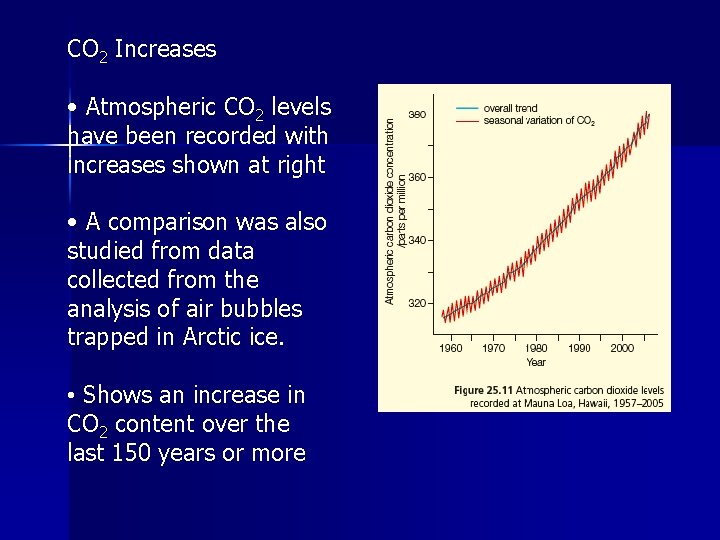
CO 2 Increases • Atmospheric CO 2 levels have been recorded with increases shown at right • A comparison was also studied from data collected from the analysis of air bubbles trapped in Arctic ice. • Shows an increase in CO 2 content over the last 150 years or more

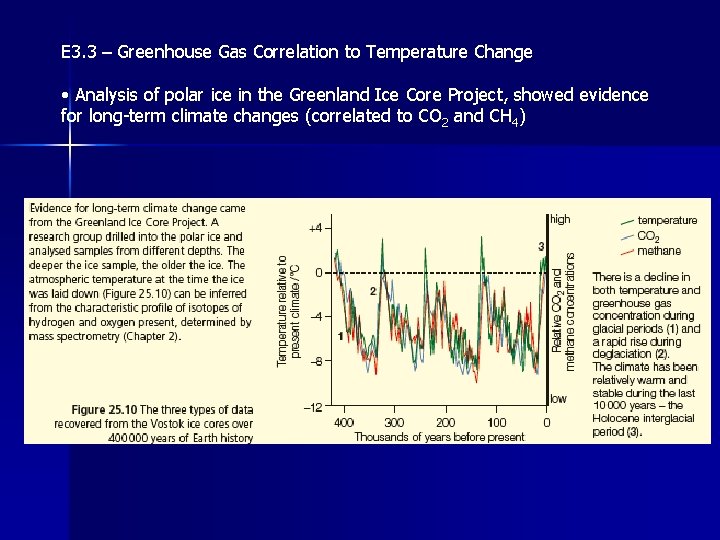
E 3. 3 – Greenhouse Gas Correlation to Temperature Change • Analysis of polar ice in the Greenland Ice Core Project, showed evidence for long-term climate changes (correlated to CO 2 and CH 4)

Enviro Chemistry Part 4 – Ozone Depletion

E 4. 1 – Ozone Formation and Depletion • Formation: O 2 + uv → 2 O • O 2 + O • → O 3 • Depletion: O 3 + uv → O 2 + O • O 3 + O • → 2 O 2

E 4. 1 – Ozone Formation and Depletion • The processes of formation and depletion of ozone in the stratosphere is normal. • The rates of formation and depletion are equal in “normal” circumstances • This delicate balance must be maintained for the ozone to be effective • Ozone Formation From O 2 and UV energy • Ozone Depletion From O 3 and UV energy

E 4. 1 – Natural Ozone Formation • Diatomic oxygen absorbs a photon with λ < 242 nm which is in the UVc region O 2 (+UV 242 nm) → O • + O • O 2 + O • + M → O 3 + M M is used as a catalyst (third body) for the reaction. It carries away excess energy.

E 4. 1 – Natural Ozone Depletion • The UV photon cause an ozone molecule to dissociate into a diatomic oxygen as well. • This process requires a photon λ = 290 -320 nm which is the UVb region O 3 (+ UV 290 -320 nm) → O 2 + O • O 3 + O • → 2 O 2 • These two processes (formation, depletion) lead to a steady concentration of ozone and UVc and UVb are largely filtered out in this process if the ideal!

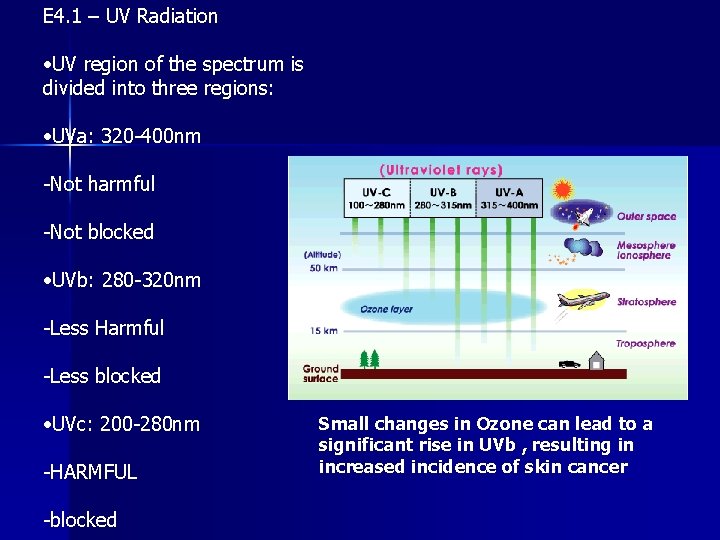
E 4. 1 – UV Radiation • UV region of the spectrum is divided into three regions: • UVa: 320 -400 nm -Not harmful -Not blocked • UVb: 280 -320 nm -Less Harmful -Less blocked • UVc: 200 -280 nm -HARMFUL -blocked Small changes in Ozone can lead to a significant rise in UVb , resulting in increased incidence of skin cancer

E 4. 1 – Ozone Layer • Ozone is a powerful oxidizing agent • When formed in the air, it’s considered a pollutant since it can reach concentrations harmful to humans and exist outside of the ozone layer • Ozone is formed by the photochemical reactants that lead to smog • The Ozone layer is considered “good” ozone as it absorbs harmful UV radiation which leads to skin cancer. Without the ozone layer, we could not have evolved • Major impacts to the ozone layer due to human activity can be seen at the polar regions

E 4. 1 – Stratospheric Ozone • The stratosphere is the layer lying above the troposphere at altitudes around 12 km(~39, 000 ft)-52 km. • Over 90% of all ozone (O 3) found in stratosphere • Exists even in ozone layer in [O 3] of <5 ppm • In the stratosphere, the level of ozone is maintained by a cyclic series of processes, in which ozone is continuously created and destroyed • These processes involve the absorption of UV

E 4. 2 – Ozone-depleting Pollutants • Examples include chlorofluorocarbons (CFCs) and oxides of nitrogen (NOx). • In 1985, the British Antarctic Survey published research showing that the ozone concentration over Antarctica was lower than expected. • This unexpected lowering of the ozone concentration became known as the “hole” • Suspected cause of the hole in the ozone was the use of CFC’s

E 4. 2 – What are CFC’s • Chlorofluorocarbons (CFC’s) were designed as propellants for aerosol sprays and as refrigerants • These chemicals are volatile and chemically inert in the troposphere which is why they were used in aerosols as they didn’t contaminate the liquid • When released at ground level, they slowly mix throughout the troposphere. They remain unreactive in the upper troposphere since UV light that can illicit a reaction is absorbed by ozone • Once the CFC’s reach the stratosphere, they react with large amounts of radiation from the sun and produce chlorine radicals Cl •

E 4. 2 – Ozone Depletion (CFC’s) • When the CFC molecules eventually reach the stratosphere, they dissociate by UV radiation: CFCl 3 + UV → CFCl 2 • + Cl • CF 2 Cl 2 + UV → CF 2 Cl • + Cl • • A chain reaction is initiated in which Cl • destroy an ozone molecule, and are then regenerated in another reaction, allowing further depletion

E 4. 2 – Ozone Depletion (N 2 O) • Most nitrogen oxides formed in the troposphere are sufficiently reactive that are used up in chemical reactions, such as those which produce smog, or acid rain. • BUT, N 2 O (nitrous oxide), is relatively unreactive allowing it to rise to the atmosphere where it can combine with a O • + N 2 O → 2 NO • • NO participates in similar chain reactions to chlorine atoms, and so is regarded as a serious ozone-depleting pollutant as well

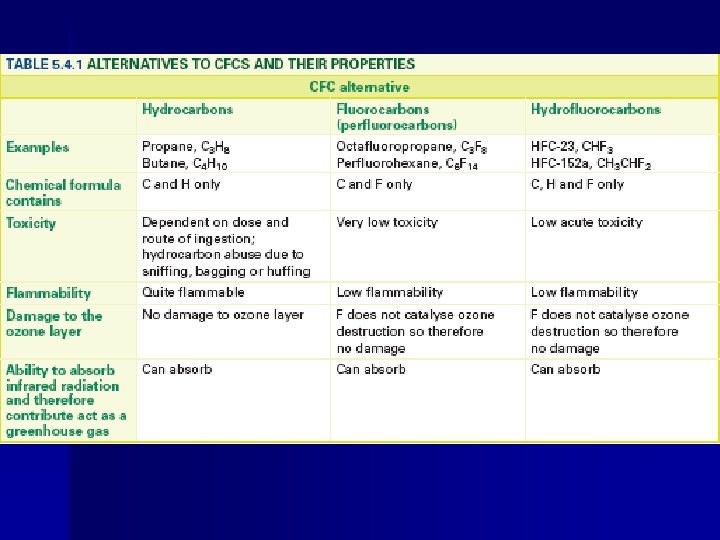
E 4. 3 – Alternatives to CFC’s • Alternatives include hydrocarbons (Cx. Hy) , fluorocarbons (FC’s) and hydrofluorocarbons (HFCs). • Include toxicity, flammability, the relative weakness of the C–Cl bond and the ability to absorb infrared radiation.

E 4. 3 – CFC Alternative (HCFC’s) • The presence of weaker C-H bonds in the molecule affects the reactivity of the molecule. - CFC’s exists as stable molecules until the stratosphere - HCFC’s react in troposphere by attack from OH • - The H-atom combines with OH • to form H 2 O and the remainder of the molecule (CFCl 2 • ) is able to break down further and release watersoluble compounds into the atmosphere which can be removed by rain

E 4. 3 – CFC Alternative (HFC’s) • Hydrofluorocarbons (HFC’s) are preferable to HCFC because they contain no chlorine. • The C-F bond is much shorter than the C-Cl bond and is thus much stronger so UV photons carry insufficient energy to break the C-F bond. • HFC’s are broken down in the troposphere in a similar way to HCFC’s, but the absence of chlorine means that HFC’s have no tendency to deplete the ozone

E 4. 3 – CFC Alternative (Cx. Hy’s) • Mixtures of propane and butane have been marketed as alternatives to CFC’s, especially in refrigeration and air conditioning applications • Appliances must be carefully designed to minimize the possibility of the hydrocarbon being ignited, for example by an electrical spark.


Enviro Chemistry Part 5 – Dissolved Oxygen in Water

E 5. 1 – Biochemical Oxygen Demand • Biochemical Oxygen Demand (BOD) describes the quantity of oxygen used when the organic material in the water is decomposed by microorganisms.
![E 5 1 O 2 The solubility of O 2g in water E 5. 1 - [O 2] • The solubility of O 2(g) in water](https://slidetodoc.com/presentation_image_h/369b2d6c12f7eb777482b94f6496e6de/image-72.jpg)
E 5. 1 - [O 2] • The solubility of O 2(g) in water is quite low � 8. 3 ppm (~0. 009 g dm-3) at 25 o. C �Solubility can be altered by changes in temperature, concentrations of dissolved materials, and quantitates of biological waste �If the [O 2] falls below 5 ppm fish will start to die �If the [O 2] falls below 3 ppm fish cannot survive

E 5. 1 – Biological Wastes • Anthropogenic Biological wastes �Human and animal waste (sewage/manure) �Food processing factory waste �Slaughterhouses �Paper mills • Organic material will gradually decay by the action of microorganisms. It consumes O 2 as: (CH 2 O)n(aq) + n. O 2(g) → n. CO 2(g) + n. H 2 O(l) (CH 2 O)n is an empirical formula representing carbohydrates such as cellulose which form the structure of plants

E 5. 1 – BOD Values • The previous process, (CH 2 O)n(aq) + n. O 2(g) → n. CO 2(g) + n. H 2 O(l), is called aerobic decomposition, as the microorganisms involved require O 2. As decay occurs, the O 2 available for other organisms such as fish is decreased • Biochemical Oxygen Demand (BOD) describes the quantity of O 2 used when the organic material in the water is decomposed by microorganisms. - Small BOD = small amount of organic matter present, water is quite pure - Large BOD = impure water, much of the O 2 present is used up in decomposition, less for fish
![E 5 1 BOD Values BOD pure water 1 ppm E 5. 1 – BOD Values • BOD [pure water] ≈ 1 ppm •](https://slidetodoc.com/presentation_image_h/369b2d6c12f7eb777482b94f6496e6de/image-75.jpg)
E 5. 1 – BOD Values • BOD [pure water] ≈ 1 ppm • BOD [polluted water] > 5 ppm • The [H 2 O] at 25 o. C ≈ 8 ppm, if the BOD is 5 ppm it lowers the [O 2] ≈ 3 ppm where it can no longer sustain fish • BOD values can be measured using a dissolved oxygen probe or via a redox titration

E 5. 2/3 - Eutrophication • Eutrophication is defined as ‘an increase in the level of chemical nutrients in an ecosystem. ’ However the terms if often used to mean the resultant increase in plant growth, lowering of [O 2] and decline of fish populations • Lake environments typically favor fish over plant life - Because levels of P and N are low, which results in limited plant growth and little decaying vegetation - Lake water has a low BOD, therefore fish populations are able to reach a high level!

E 5. 3 – Algal Bloom • Human activities can lead to increases of nutrients in rivers and streams. - Use of fertilizers on farmland - Release of sewage onto rivers • Both result in a large increase in [nutrient], and phosphorus in particular in the water they feed • This excess of nutrients leads to excess growth of primitive plants called algae, which float on the surface of the water, a ‘green scum’ known as an algal bloom - The turbidity (cloudy) in the water increases

Eutrophic lakes can be recognized by a green tint to the water

Algae blooms result in unpleasant tasting water and may release harmful toxins even • The color, taste, and toxicity of the water affect human activities such as fishing and boating • Water clogged with algae is more difficult to treat for drinking • Biggest problem is AFTER the algae die - Dead algae is consumed by aerobic bacteria which use up the dissolved O 2 in the water - [O 2] falls below necessary level, only material like sludgeworms can survive. Lake is nearly useless for fishing and has an effect on local communities
![After the algal bloom O 2 falls so low that aerobic bacteria cannot survive After the algal bloom, [O 2] falls so low that aerobic bacteria cannot survive](https://slidetodoc.com/presentation_image_h/369b2d6c12f7eb777482b94f6496e6de/image-80.jpg)
After the algal bloom, [O 2] falls so low that aerobic bacteria cannot survive • Instead, anaerobic bacteria decompose the remaining dead algae, forming foulsmelling products such as ammonia, hydrogen sulfide, methane and thioalcohols • Bacteria may release dangerous toxins lethal to animals

E 5. 4 – Thermal Pollution • The temperature of water highly affects the solubility of gases. • At 25 o. C the solubility is 8. 3 ppm • At 30 o. C this falls to 5 ppm when fish begin to die

Human activities can lead to an increase in water temperature known as thermal pollution • This occurs near power stations where water is drawn to cool the steam from the turbines in devices called heat exchangers. When water leaves it could be 20 o. C warmer • [O 2] is decreased when temperature rises because • Solubility of O 2 is decreased with lower Temps • Microorganisms respire more quickly

High temperatures also cause enzymes in microorganisms to denature and as a result they cannot digest their food molecules • Changing temperatures may also alter the cycle of fish such as: • Spawn (lay eggs) earlier • Eggs hatch earlier • At this time in the cycle the necessary nutrients may not be available in the system • Thermal pollution may also be cooling due to cold water at the bottom of reservoirs released into streams below

Enviro Chemistry Part 6 – Water Treatment

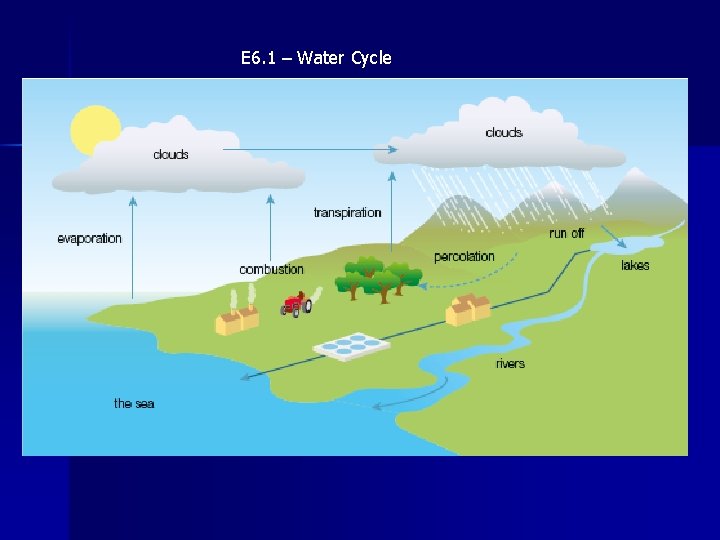
E 6. 1 – Water Cycle

E 6. 1 – Water and its Pollutants • Water is an excellent solvent and dissolves many different chemical substances • As water falls through the atmosphere it dissolves gases, such as CO 2, and pollutants such as SO 2 and NOx. • Chemical fertilizers washed off the farmland will add NO 3 - and PO 43 - ions • River water may contain pesticides, bacteria and oil • All of these impurities must be removed before it can be used for drinking

E 6. 1 – Types of Water Pollutants • Water pollution falls into two broad categories: �Point sources – occurs when the source of pollution is clearly identifiable at one point For example: a chemical factory releasing toxic substances into a river �Non-point sources – describes situations in which water collects pollutants over a larger area, and thus cannot be attributed to a single source Examples: Acid rain polluting a lake, fertilizer run-off which accumulates N and P in compounds in rivers

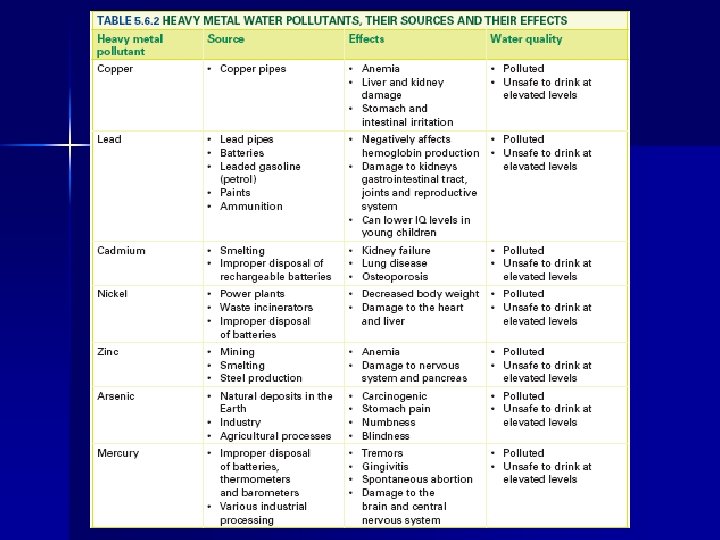
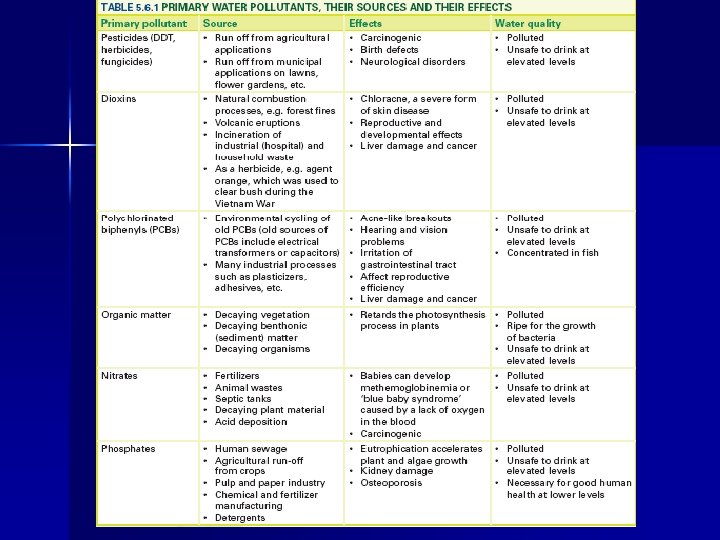
The primary pollutants found in waste water are: �Heavy metals (toxic metals) �Pesticides (insecticides and herbicides) �Chemical wastes (dioxins, PCB’s) �Organic Wastes (sewage) �Fertilizers



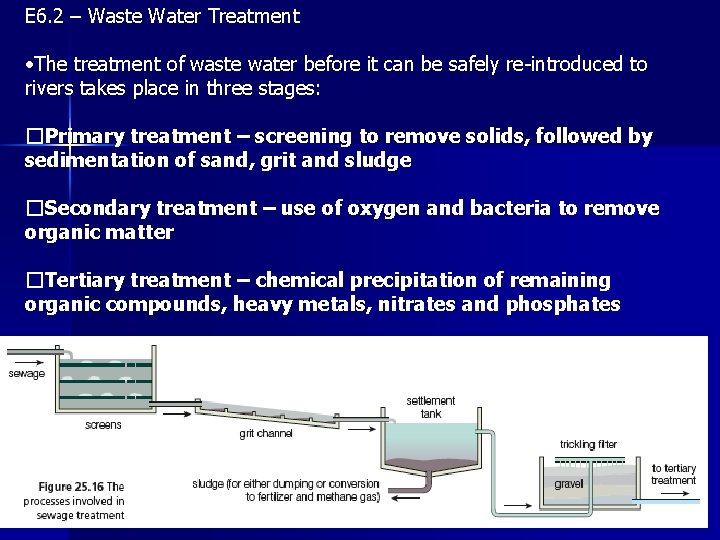
E 6. 2 – Waste Water Treatment • The treatment of waste water before it can be safely re-introduced to rivers takes place in three stages: �Primary treatment – screening to remove solids, followed by sedimentation of sand, grit and sludge �Secondary treatment – use of oxygen and bacteria to remove organic matter �Tertiary treatment – chemical precipitation of remaining organic compounds, heavy metals, nitrates and phosphates

E 6. 2 – Primary Treatment • Water is sent through coarse mesh screens: - Raw waste water contains floating and suspended solid material. This may include paper, rags, wood and plastic rubbish that may cause blockage elsewhere in the treatment plant. • Water is passed through grit channel - The speed of the water is slowed so sand grit settle out. A spinning centrifuge could also be used. • Water is then passed into large holding tanks to settle - Fine solid particles form sludge at bottom, insoluble grease floats to top and is skimmed off • Sometimes this treatment alone is enough

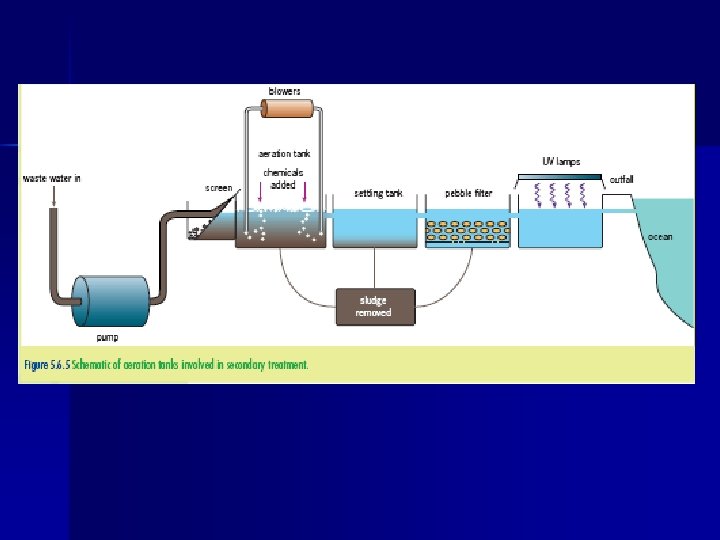
E 6. 2 – Secondary Treatment • Secondary treatment focuses on lowering BOD by removing organic matter • The principal secondary treatment involves allowing aerobic bacteria to oxidize the organic matter - One method involves pouring of the waste over a bed of small stones covered in microorganisms - More effective method called the activated sludge process. Bacteria and sewage are mixed and blasted with air allowing for the bacteria to multiply rapidly and feed on the organic material. This process removes 90% of the BOD from waste water


B 6. 2 – Tertiary Treatment • Tertiary treatment is sometimes called advanced water treatment and removes the remaining inorganic pollutants from the water (PO 43 -, N- complexes, heavy metal ions). • This process is very important where industrial point sources such as metal works or chemical plants have led to high [pollutants] • Nitrogen compounds are removed so they do not contribute to eutrophication of rivers and lakes. There are two common types (ammonium ions, and nitrate ions. )

E 6. 2 – Tertiary, NH 4+ removal • In the tertiary treatment, water is first treated with nitrifying bacteria, which oxidize the NH 4+ ions to nitrate ions • This is a two-step process: NH 4+(aq) + 1½ O 2(g) → NO 2 -(aq) + 2 H+(aq) + H 2 O(l) NO 2 -(aq) + ½ O 2(g) → NO 3 -(aq)

E 6. 2 – Tertiary, NO 3 - removal • The nitrate ions originally present in waste water, along with those formed in the treatment of NH 4+, are then reduced (by different strains of bacteria) to nitrogen gas, which is released into the atmosphere • 2 NO 3−(aq) + 10 e− + 12 H+(aq) → N 2(g) + 6 H 2 O(l)

E 6. 2 – Tertiary, PO 43 - removal • Phosphates are removed by either biological or chemical processes. • Water treated with biomass containing specific bacteria which absorb the PO 43 - ions, and the product can later be used as fertilizer • Alternatively, PO 43 - ions can be removed by chemical precipitation Fe 3+ (aq) + PO 43 -(aq) → Fe. PO 4(s) Al 3+ (aq) + PO 43 -(aq) → Al. PO 4(s)

E 6. 2 – Tertiary, Heavy Metal removal • Heavy metals can be removed by chemical precipitation or ion exchange. • Most transition metal ions have insoluble hydroxides, so Ca(OH)2 or Na 2 CO 3 are added Cr 3+ (aq) + OH-(aq) → Cr(OH)3(s) • A coagulant can then be added to clump and collect insoluble particles together • Ion exchange resin is a material that binds reversibly to particular cations or anions as they are more attracted to heavy metals than original ions 2 Na+(resin) + Cd 2+ (aq) ⇌ 2 Na+(aq) + Cd 2+ (resin)

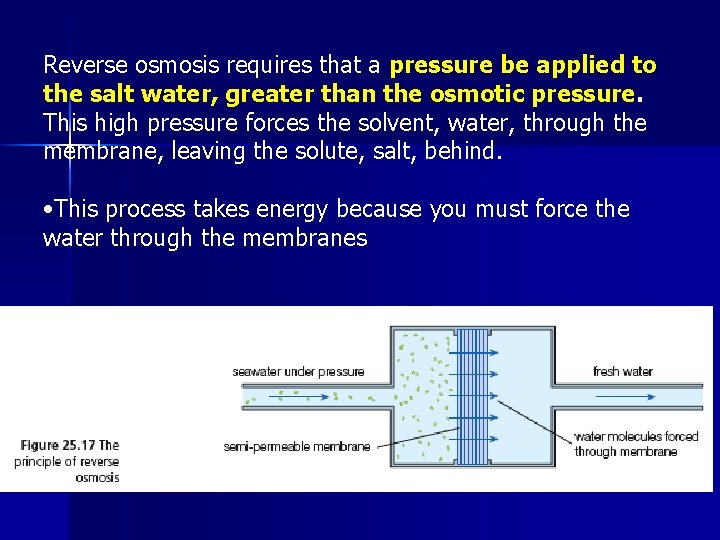
E 6. 3 – Fresh water from the Sea • Osmosis is a natural process which serves to equalize the concentrations of solutions. • If two solutions (one salt water, one water) are placed on two sides of a semi-permeable membrane, the water will tend to flow to the salt solution side until concentrations are in equilibrium • If pure water is desired, we want the opposite and the solution must flow against the osmotic flow. This process is called reverse osmosis.

Reverse osmosis requires that a pressure be applied to the salt water, greater than the osmotic pressure. This high pressure forces the solvent, water, through the membrane, leaving the solute, salt, behind. • This process takes energy because you must force the water through the membranes

E 6. 3 – Thermal Desalination • The most commonly used method to date is multi-stage flash distillation (MSF). • Sea water is heated under high pressure and then passed into a chamber at lower pressure : - Rapid decrease in pressure causes water to ‘flash’ evaporate - After each ‘flash’ the steam is cooled in another chamber - The cycle takes advantage of the steam to heat more water, keeping energy consumption minimized

E 6. 3 – Advantage of Different Methods of Attaining Fresh Water

Enviro Chemistry Part 7 – Soil

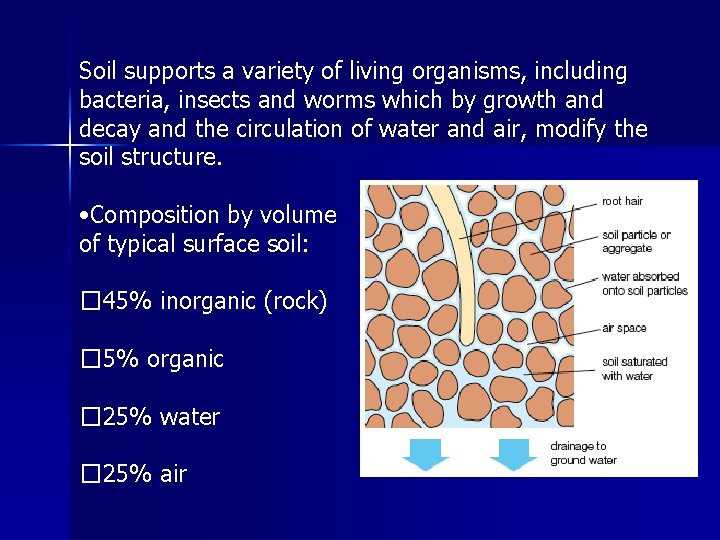
Soil is composed of organic and inorganic components in varying proportions • Organic component is called the humus and consists of plant material that has been partly decayed by bacteria and fungi • Inorganic component includes mineral particles (gravel, sand, silt, and clay), water, and air. • Water trapped between soil grains contains ions (from dissolving of minerals) which can be absorbed by plant roots. • This liquid containing dissolved ions and organic substances, is called the soil solution

Soil supports a variety of living organisms, including bacteria, insects and worms which by growth and decay and the circulation of water and air, modify the soil structure. • Composition by volume of typical surface soil: � 45% inorganic (rock) � 5% organic � 25% water � 25% air

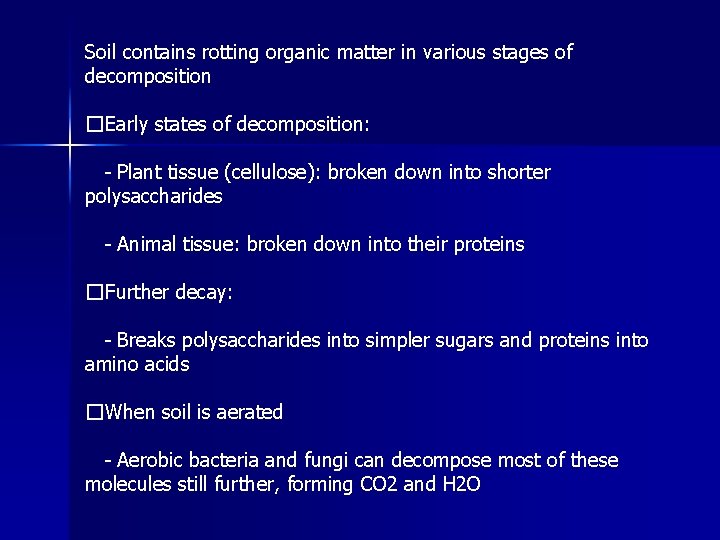
Soil contains rotting organic matter in various stages of decomposition �Early states of decomposition: - Plant tissue (cellulose): broken down into shorter polysaccharides - Animal tissue: broken down into their proteins �Further decay: - Breaks polysaccharides into simpler sugars and proteins into amino acids �When soil is aerated - Aerobic bacteria and fungi can decompose most of these molecules still further, forming CO 2 and H 2 O

Soil Organic Matter (SOM) is beneficial to soil in the following ways: �Products of partial decomposition replenish the soil by turning large molecules to smaller ones and soluble ones which plant roots can take up �Partially decayed organic material helps to hold soil together. When soil is bound together, water (and thermal) retention is improved �Dark-colored SOM-rich soil absorbs heat better than lighter-colored (low SOM) soil

In wet soils, O 2 is less able to reach the organic material. As a result, anaerobic bacteria take over, and form more complex organic compounds such as phenols and carboxylic acids � These are ‘humic substances’ and the mixture is called humus � In cold/wet climates. Up to 95% organic matter known as ‘peat soils. ’ • The phenols and carboxylic acids in the humus (weak acids) help to buffer the soil p. H � Important since p. H affects the solubility of metal cations in the soil

Much of the SOM is insoluble in water but helps to retain water and water-soluble plant nutrients so that they can be taken up by plants roots. Nutrients include inorganic minerals such as phosphorus, nitrogen and sulfur, which are needed for healthy plant growth. SOM also has many other important physical functions. There are spaces or pores that exist between the SOM which act to hold water (much like a sponge), oxygen and nitrogen. Some of the SOM along with fungi create aggregates that allow for a more stable structure of the soil. This creates a soil layer that is more resistant to erosion; therefore, soils with greater amounts of SOM have less degradation.

Soil degradation takes place when actions result in the soil being unhealthy or infertile. This degradation can take place naturally as well as a result of human activity. Natural sources of soil degradation include wind and water erosion. The rates of these types of erosion can be increased by improper land use practices. - For example, when small shrubs are removed to make way for farming, the roots that once held soil in place are no longer there, which means that wind and water erosion increase and valuable soil is lost. The three main anthropogenic forms of soil degradation are salinization, nutrient depletion, and soil pollution.

Salinization: �The build-up of salts in the soil often becoming toxic to plants. �Can arise from : - Irrigation: water is diverted from waterways to farmland to support crops where rainfall is limited. The irrigation water may contain some dissolved salts. - The natural water table �The water transports salts to the soil as they are dissolved. As the water evaporates, the salt is left behind and can build up over time.

Nutrient Depletion �The nitrogen cycle requires that the minerals taken up during plant growth will re-enter the soil when the plants die and decay. �If, through cultivation and harvesting, crops are continuously removed, the nutrients and minerals go along with them. �Nutrient depletion can be avoided by crop rotation over a number of years (and some off years where the crops are plowed back into the soil for nutrients).

Soil Pollution Soil nutrient depletion is often addressed by the use of chemical fertilizers which contain nitrates (NO 3 -) and phosphates (PO 43 -) • These excess nutrients can lead to environmental issues on their own • As well as inorganic pollutants, organics can be soil pollutants as well.

Soil pollution can also occur when harmful air pollutants settle onto the topsoil, get turned under and contaminate the soil food web. These contaminants can be released into our water supplies and back into the atmosphere in the form of particulate matter. Soil pollution can also result when hazardous waste from industry is placed in landfills and leaches into the surrounding soil.


Enviro Chemistry Part 8 – Waste

Anything you throw away in your rubbish bin is called waste. As the world population and the standard of living increases, larger and larger amounts of waste are produced. Once your garbage is driven away in the garbage truck there are two main ways in which it is: � Landfill sites (burying the waste) �Incineration (burning the waste) - Both methods are potentially damaging �Recycling waste is advantageous because it removes the need to dispose of waste and cuts down on the need for non-renewable resources

Solid Waste falls into five categories �Food and kitchen waste and plant waste – biodegradable �Recyclable materials – glass, plastic, paper, etc �Composite wastes – mixtures of materials such as clothing and packaging materials – difficult to recycle �Inert wastes – rubble, debris, etc �Hazardous wastes – paints, garden chemicals, batteries, light bulbs, medicines

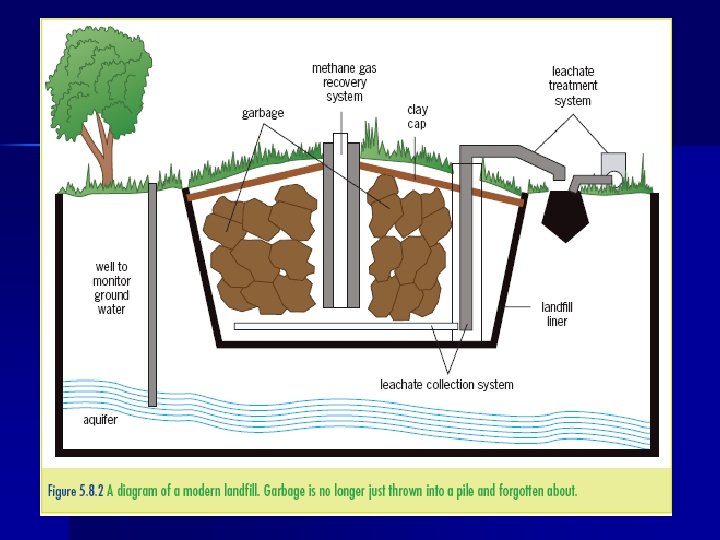
E 8. 1 - Landfill • Landfills are designed to limit surface water (rain, runoff, etc) from passing through and leaching out harmful materials. • Sites chosen are often made of clay – a low permeable soil so ground water movement is small • Pits are lined with plastic, gravel, and a drainage system �This ensures that polluted water that accumulates (leachate) can be collected and disposed of properly �When full, the site is covered with the same layer to keep rain water out.


When waste is added to site �At first, when O 2 is present, aerobic bacteria work on the organic material producing CO 2 �When O 2 used up, anaerobic decomposition occurs and generates CH 4, H 2 S, and H 2. - These gases can accumulate below ground so an ventilation system must be constructed - Air can be pumped into the landfill in order to increase the aerobic bacteria decomposition

The liquid leachate can contain heavy metals and cyanide and must be extracted from the landfill via the drainage system. • The liquid must be treated by chemical or biological means to remove pollutants before re-entering waterways (discussed previously) - Heavy metal precipitation (E 6) • The cyanide can be removed by oxidation with sodium chlorate Na. CN + Na. OCl → Na. CNO + Na. Cl 2 Na. CNO + 3 Na. OCl + H 2 O → 3 Na. Cl + N 2 + 2 Na. HCO 3

E 8. 1 - Incineration • Incineration has many advantages - Removes bulk from solid waste - Resultant ash has a uniform composition that can be more densely packed into landfills - Can be burned for power generation • However, the flu gas must be thoroughly cleaned before being allowed to enter the environment as it contains CO, HCl, HCN, organics, heavy metals, and particulates - Two types of incinerators are the Rotary-kiln and Fluidized-bed.

Rotary-kiln Incinerator • Rotating chambers are used to allow movement of waste and to ensure that all of the waste is exposed to air. • Most of the waste is combusted to form gases �Passed to ‘afterburner’ to ensure that solid particulates are fully combusted �Gases are then ‘scrubbed’ to remove pollutants • Remaining solid waste drops out of the kiln and is cooled with water, recyclable materials can be reclaimed from this solid waste.

Fluidized-bed Incinerator • A sand bed is used to allow hot air to be basted in. �Separates the sand grains, allowing air between them �The sand is suspended on the air currents and it behaves like a fluid, flowing and circulating. �Ground-up waste is introduced to the sand bed where it’s suspended and the air is mixed throughout ensuring that maximum surface area of the waste particles is exposed to air for combustion

Incinerator Air Pollution • Most of the carbon present in waste (mostly in organic material and plastics) is converted into CO 2 in the incinerator. • This is arguably preferable to landfills where methane is formed. �If the methane is not reclaimed as fuel it enters the atmosphere as a green house gas with a very high GWP (higher than CO 2) • Other flu gas pollutants are removed in procedures described in E 1.

Recycling One of the best ways to minimize the influence waste has on our environment and to provide a sustainable environment is to recycle. Recycling can be an expensive way of dealing with problematic waste and not all waste can be recycled; separating the different recyclables can be time consuming and difficult.


E 8. 3/4 – Radioactive Wastes Radioactivity is the release of radiation from the nucleus of an atom as it changes, or decays, into a different element. �Nuclei decay in order to stabilize their structure �Reduces neutron: proton ratio in nucleus �Three types: alpha, beta, gamma

Nuclear power plants have many advantages over other sources of energy. They do not contribute to air pollution or greenhouse gases, and their fuel source, uranium, is abundant enough to supply energy for the next 1000 years. One of the major problems of nuclear power plants is the disposal of the spent fuel rods that provided the energy for the nuclear fission reactor. These spent fuel rods are sources of high-level waste (HLW) because they give off large amounts of ionizing radiation for a long time. These spent fuel rods must be cooled for several years in deep pools inside the plant or in special shielded storage facilities at another site. It is said that this waste must be stored for tens of thousands of years before it can be disposed of safely.

A second category of radioactive waste is low-level waste (LLW), which refers to waste that gives off small amounts of ionizing radiation for a short amount of time. Low-level waste is waste that may have come into contact with radioactive substances directly and therefore has become contaminated. It includes radioactively contaminated industrial or research waste such as paper, rags, plastic bags, rubber gloves, protective clothing and packaging material. Hospitals, medical schools and radiopharmaceutical schools all produce large amounts of LLW. This waste can be placed in steel drums and buried in landfills.

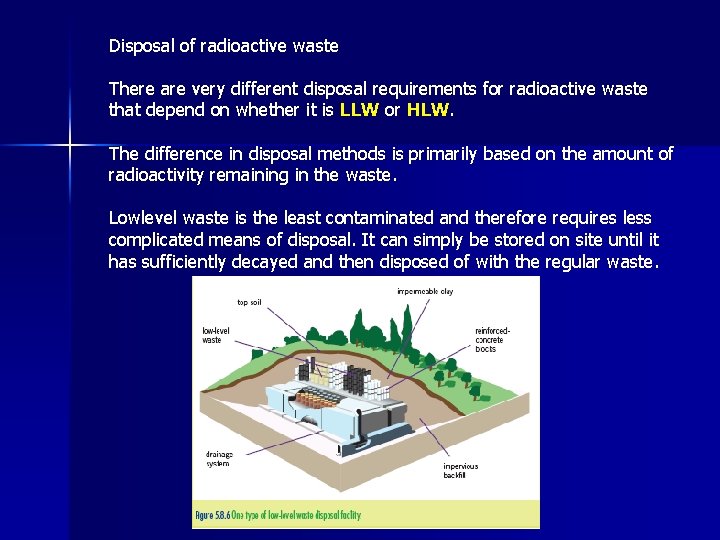
Disposal of radioactive waste There are very different disposal requirements for radioactive waste that depend on whether it is LLW or HLW. The difference in disposal methods is primarily based on the amount of radioactivity remaining in the waste. Lowlevel waste is the least contaminated and therefore requires less complicated means of disposal. It can simply be stored on site until it has sufficiently decayed and then disposed of with the regular waste.

The spent fuel that is used to fuel a nuclear power plant is initially stored in deep pools of water on the nuclear power plant site. The water in these pools acts to absorb the heat energy that is released by the spent fuel and to protect the workers from the radiation. These spent fuel rods are then reprocessed to recover the unfissioned uranium ore. This liquid waste is then classified as HLW and must be disposed of safely.

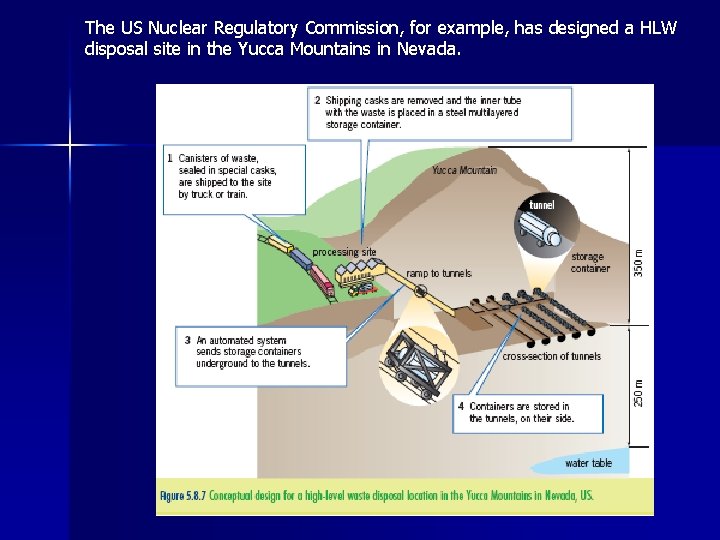
The US Nuclear Regulatory Commission, for example, has designed a HLW disposal site in the Yucca Mountains in Nevada.

Nuclear Medical Waste • Radioactive isotopes are used in numerous medical applications �Medicines or diagnostic tools – short ½ life substances are used. Technetium is used as a tracer. Iodine-131 is used to treat thyroid cancers (since the gland absorbs iodine) �Radiotherapy – long ½ life substances like cesium-137 are used to generate radiation in machines used for radiotherapy �X-rays for radiography – long ½ life substances such as cobalt-60 and iridium-192 are used to generate X-rays for imagine • Medical gloves and clothing are considered low-level, isotopes from defunct X-ray machines are considered high-level

Topics E 9 -E 12 are HL material only! Enviro Chemistry Part 9 – Further Ozone for HL

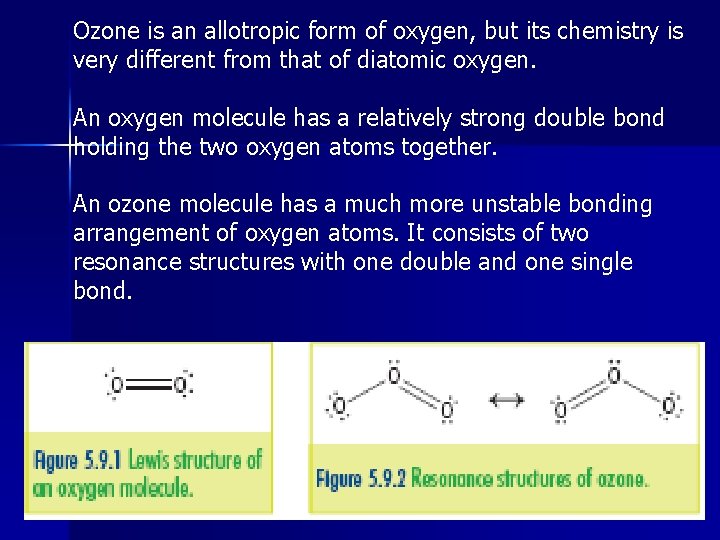
Ozone is an allotropic form of oxygen, but its chemistry is very different from that of diatomic oxygen. An oxygen molecule has a relatively strong double bond holding the two oxygen atoms together. An ozone molecule has a much more unstable bonding arrangement of oxygen atoms. It consists of two resonance structures with one double and one single bond.

The bond between the oxygen atoms in O 2 is shorter (121 pm) than that of the oxygen–oxygen bond in ozone (128 pm). The longer oxygen–oxygen bond length indicates that the oxygen–oxygen bond in ozone is weaker than that in O 2. Light of shorter wavelength, and thus higher energy, is needed to break the bond in a molecule of oxygen than in ozone.

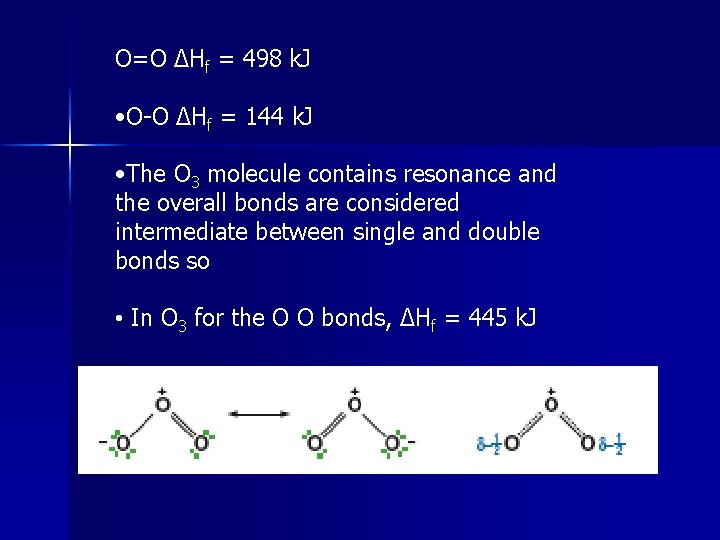
O=O ΔHf = 498 k. J • O-O ΔHf = 144 k. J • The O 3 molecule contains resonance and the overall bonds are considered intermediate between single and double bonds so • In O 3 for the O O bonds, ΔHf = 445 k. J

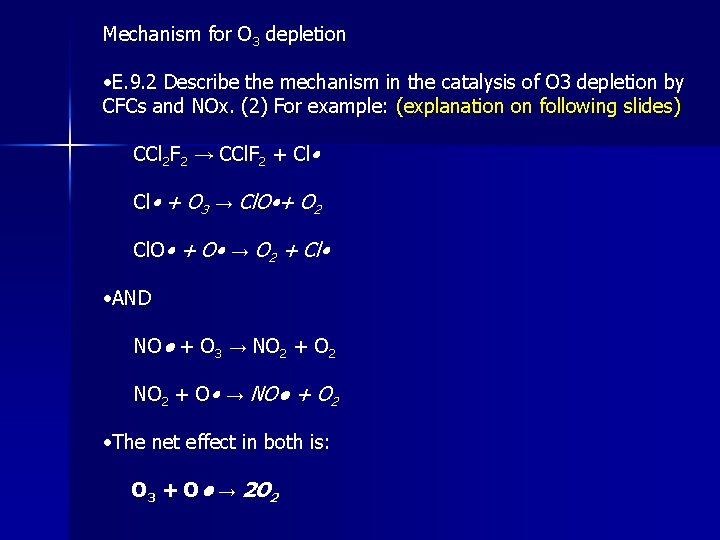
Mechanism for O 3 depletion • E. 9. 2 Describe the mechanism in the catalysis of O 3 depletion by CFCs and NOx. (2) For example: (explanation on following slides) CCl 2 F 2 → CCl. F 2 + Cl • + O 3 → Cl. O • + O 2 Cl. O • + O • → O 2 + Cl • • AND NO● + O 3 → NO 2 + O 2 NO 2 + O • → NO● + O 2 • The net effect in both is: O 3 + O • → 2 O 2

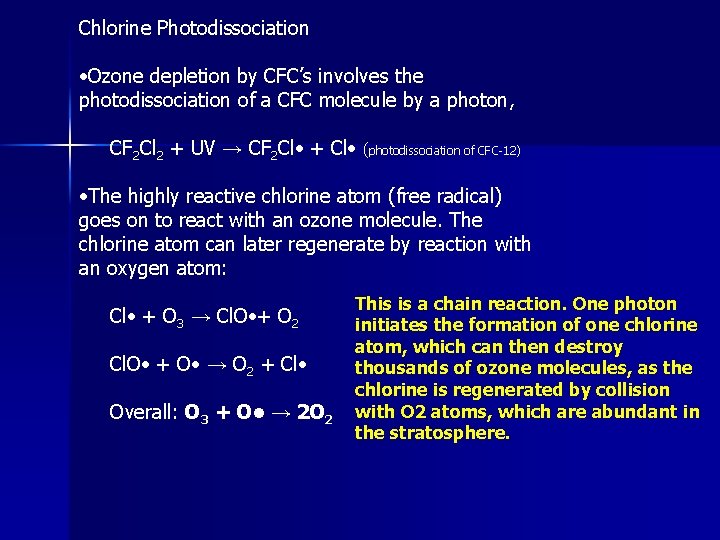
Chlorine Photodissociation • Ozone depletion by CFC’s involves the photodissociation of a CFC molecule by a photon, CF 2 Cl 2 + UV → CF 2 Cl • + Cl • (photodissociation of CFC-12) • The highly reactive chlorine atom (free radical) goes on to react with an ozone molecule. The chlorine atom can later regenerate by reaction with an oxygen atom: Cl • + O 3 → Cl. O • + O 2 Cl. O • + O • → O 2 + Cl • Overall: O 3 + O • → 2 O 2 This is a chain reaction. One photon initiates the formation of one chlorine atom, which can then destroy thousands of ozone molecules, as the chlorine is regenerated by collision with O 2 atoms, which are abundant in the stratosphere.

N 2 O Ozone Depletion • As discussed earlier (part 04) nitrogen monoxide, NO (exists as radical), is formed in the stratosphere by the reaction of nitrous oxide, N 2 O, with an oxygen atom N 2 O + O • → 2 NO • • Nitrogen monoxide can deplete ozone as follows: • NO + O 3 → 2 NO 2 + O 2 NO 2 + O • → NO • + O 2 • Again, the nitrogen monoxide has been regenerated and can continue depleting more ozone molecules.

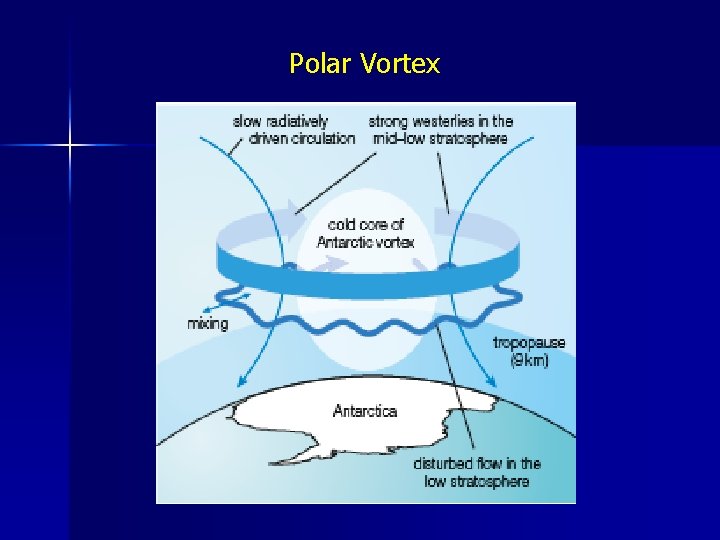
Polar Ozone Most CFC’s are released in the tropical and temperate regions, where most of the Earth’s population live • However, ozone layer damage has been confined to the polar regions Long-term studies of [O 3] suggest that depletion is fastest in the spring months -September-October in the Antarctic -March-April in the Arctic • The polar regions are naturally colder than the tropics, and in addition, air currents in the stratosphere tend to isolate a region of air above the poles so it does not mix with the tropical air during the polar winter. • This results in very low temperatures in the polar stratosphere, -80 o. C. This is termed the polar vortex

Polar Vortex

Polar Stratospheric Clouds • These low temps result in high-altitude polar stratospheric clouds (PSC’s) • H 2 O and HNO 3 condense on pre-existing microparticles on sulfur (S) containing compounds. (at low T’s form ice crystals) • The existence of these clouds (PSC’s) along with the isolation of gas at the poles, causes a shift in the chlorine containing compounds that occurs: • Instead of HCl and Cl. ONO 2, species such as Cl 2 appear • This occurs bc the cloud particles offer a catalytic surface which speeds up gas phase reactions and through the winter the PSC reactions build up Cl 2

Remember, ozone depletion is caused by UV radiation. • In the winter, the extreme polar latitudes receive almost not sunlight so limited depletion • In the spring, the Sun reappears and photolysis the Cl 2(g) (favored chlorine containing compound) that have built up through the winter to form Cl • , which enter the ozone depletion cycle and cause a sudden rapid depletion of ozone

In summer, the temperatures rise and the polar vortex is no longer in effect and the PSC’s disperse • The balance in chlorine compounds shifts back to more stable (unreactive) compounds. • The ozone depletion stops and the hole has a chance to rebuild itself through photochemical reactions • The hole also is rebuilt by finally mixing with ozonerich air from higher latitudes through the summer. • Compounds favored are HCl and Cl. ONO 2

Enviro Chemistry Part 10 – Smog for HL

Originally smog referred to fog-like covering that fell over cities due to large amounts of smoke and SO 2 from the burning of coal. • Today, the term generally refers to photochemical smog in which primary pollutants derived from vehicle traffic undergo a series of light-driven chemical reactions forming toxic compounds

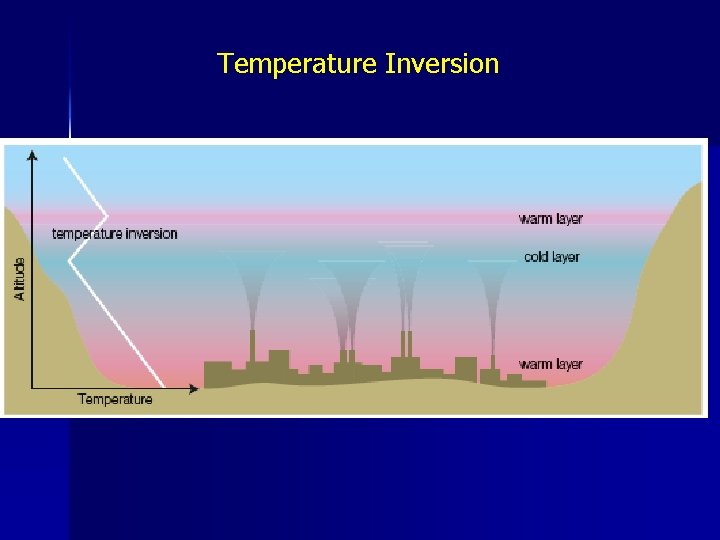
Conditions Leading to Photochemical Smog • The primary pollutants leading to photochemical smog are NO and VOC’s from vehicle exhausts - N 2 + O 2 → 2 NO • • Geographical location such as L. A. and Mexico City both located in-between mountains for a geological ‘bowl’ or ‘basin’ • These cities also have inversion layers (temperature inversion) in which the air close to the ground is colder than the above which prevents the polluted air from the city from rising • The build-up of pollutants are then acted upon by sunlight

Temperature Inversion

Cities that experience intense photochemical smog include Athens, Hong Kong, Houston, Los Angeles, Mexico City, Sao Paulo, and Tehran. • Photochemical Smog - Primary Pollutants: -NOx(NO + NO 2), VOC’s, CO, particulate matter - Secondary Pollutants: - O 2, NO 2, H 2 O 2, PAN (peroxyacetyl nitrate), partially oxidized VOC’s, HNO 3, particulate matter

Formation of Smog Pollutants Harmful substances from photochemical smog include NO 2, O 3, aldehydes (HCHO), peroxyethanoyl nitrate (PAN’s), and secondary aerosol particles (comprised of sulfates, nitrates and oxidized organic compounds). • All from free-radical processes driven by the sun

Formation of NO 2 and Ozone • Formation of NO 2 2 NO • + O 2 → NO 2 • Formation of ozone NO 2 • + UV (430 nm) → NO • + O 2 + M → O 3 + M NO • + O 3 → NO 2 • + O 2 • If these were the only processes involved, then a steady state would be reached, with ozone depletion/formation stable. BUT, further processes allow NO to re-oxidize to NO 2 without depleting ozone. More ozone created than destroyed = ozone build up!

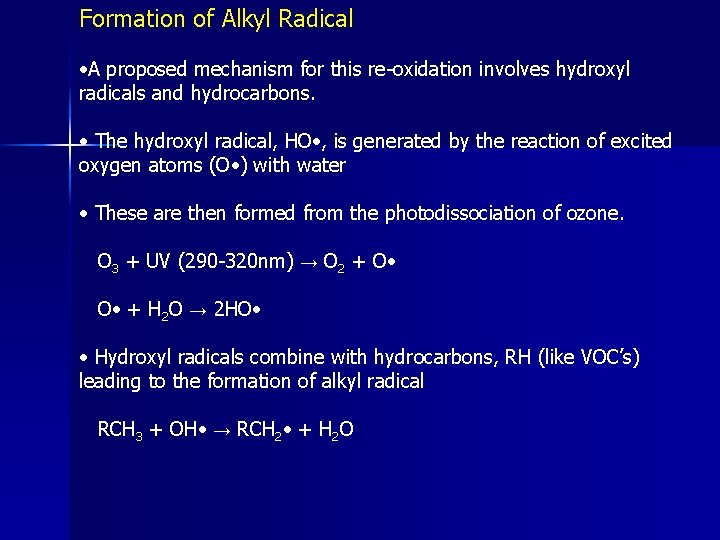
Formation of Alkyl Radical • A proposed mechanism for this re-oxidation involves hydroxyl radicals and hydrocarbons. • The hydroxyl radical, HO • , is generated by the reaction of excited oxygen atoms (O • ) with water • These are then formed from the photodissociation of ozone. O 3 + UV (290 -320 nm) → O 2 + O • + H 2 O → 2 HO • • Hydroxyl radicals combine with hydrocarbons, RH (like VOC’s) leading to the formation of alkyl radical RCH 3 + OH • → RCH 2 • + H 2 O

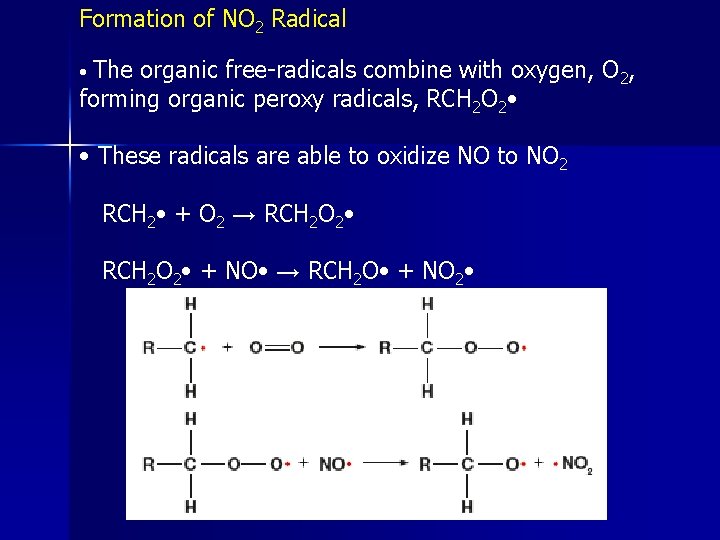
Formation of NO 2 Radical The organic free-radicals combine with oxygen, O 2, forming organic peroxy radicals, RCH 2 O 2 • • • These radicals are able to oxidize NO to NO 2 RCH 2 • + O 2 → RCH 2 O 2 • + NO • → RCH 2 O • + NO 2 •

The resulting RCH 2 O • radicals can react with oxygen molecules to form aldehydes, RCHO : RCH 2 O • + O 2 → RCHO + HO 2 • • HO 2 + NO • → HO • + • NO 2 • These radicals convert NO to NO 2, which then photolyzes to form ozone • OH + RCH 3 + 2 O 2 + 2 NO • → H 2 O + RCHO + 2 NO 2 • + • OH • Combined with 2 NO 2 + 2 O 2 → 2 NO • + 2 O 3 • Results in: • OH + RCH 3 (need NO + O 2) → H 2 O + RCHO + 2 O 3 + • OH

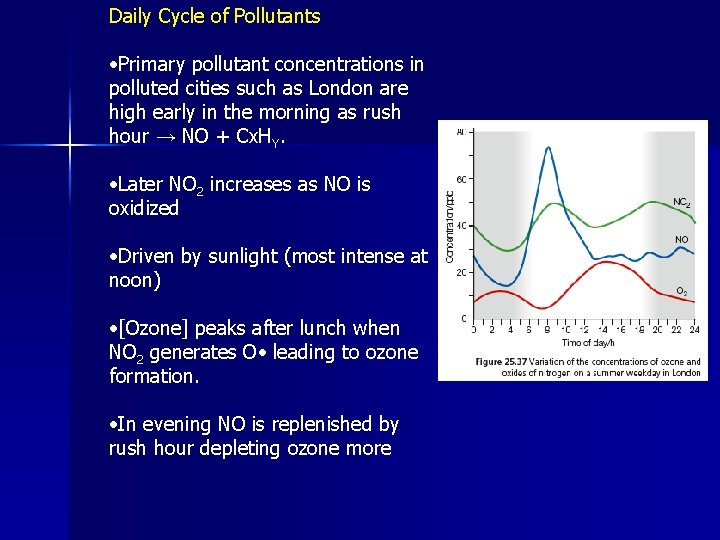
Daily Cycle of Pollutants • Primary pollutant concentrations in polluted cities such as London are high early in the morning as rush hour → NO + Cx. HY. • Later NO 2 increases as NO is oxidized • Driven by sunlight (most intense at noon) • [Ozone] peaks after lunch when NO 2 generates O • leading to ozone formation. • In evening NO is replenished by rush hour depleting ozone more

Harmful Effects of Ozone • Contained within many synthetic materials, C=C can often be found. - These compounds are paints, dyes, and plastics - The addition of O 3 across this double bond can cause deterioration and color bleaching - Polymer chains in rubber tires could be broken down by ozone, causing them to crack and split - O 3 attacks green plants, discoloring their leaves and resulting in decreased photosynthesis

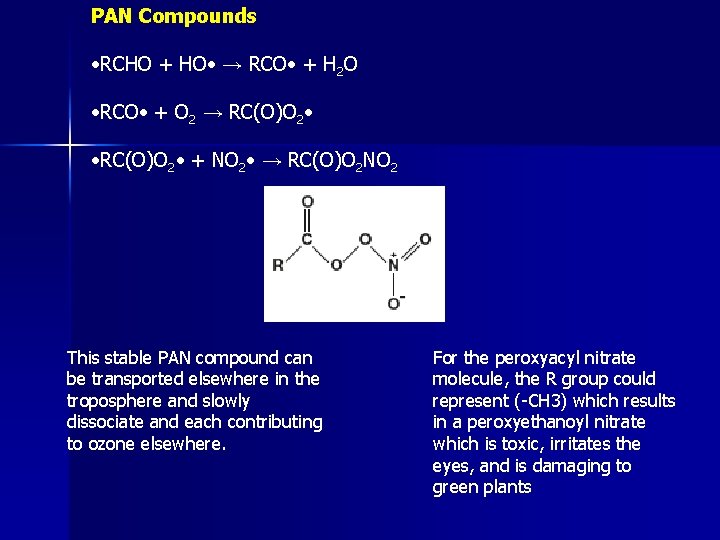
Formation of PANs • Remember, PANs are harmful substances that results from photochemical smog • As we have seen NO 2 shows up in O 3 formation equations • NO 2 can be removed from the photochemical smog chain reaction by reaction with the PAN (peroxyacyl) radical. The resultant compound, peroxyacyl nitrate, has many adverse health effects

PAN Compounds • RCHO + HO • → RCO • + H 2 O • RCO • + O 2 → RC(O)O 2 • • RC(O)O 2 • + NO 2 • → RC(O)O 2 NO 2 This stable PAN compound can be transported elsewhere in the troposphere and slowly dissociate and each contributing to ozone elsewhere. For the peroxyacyl nitrate molecule, the R group could represent (-CH 3) which results in a peroxyethanoyl nitrate which is toxic, irritates the eyes, and is damaging to green plants

Enviro Chemistry Part 11 – Further Acid Deposition HL

Creating OH • • As discussed earlier O • + H 2 O → 2 HO • • The HO • is also formed by: O 3 + H 2 O → 2 HO • + O 2 • Aside from their role in formation of photochemical smog, the HO • are important in the formation of nitric, nitrous, and sulfuric acid in the atmosphere

Acid Deposition by NO and SOx Formation of hydroxyl radicals: H 2 O+O 3 → 2 HO • +O 2 OR… H 2 O +O • → 2 HO • + NO 2 → HNO 3 HO • + NO → HNO 2 HO • + SO 2 → HOSO 2 • + O 2 → HO 2 • + SO 3 (SO 3 + H 2 O → H 2 SO 4)

Formation of N Acids • N 2 + O 2 → 2 NO (from combustion of fuel) • Formation of Nitrous Acid (HNO 2) HO • + NO • → HNO 2 Moderately soluble in water, so HNO 2 dissociates HNO 2 + H 2 O ⇌ H 3 O+ + NO 2 - (weak acid dissociates) HNO 2 (+UV) → HO • + NO (photolyzed back to NO, HO) • Formation of Nitric Acid (HNO 3) 2 NO • + O 2 → 2 NO 2 • HO • + • NO 2 → HNO 3 Strong Acid = more soluble in water HNO 3 + H 2 O → H 3 O+ + NO 3 - (100% dissociation)

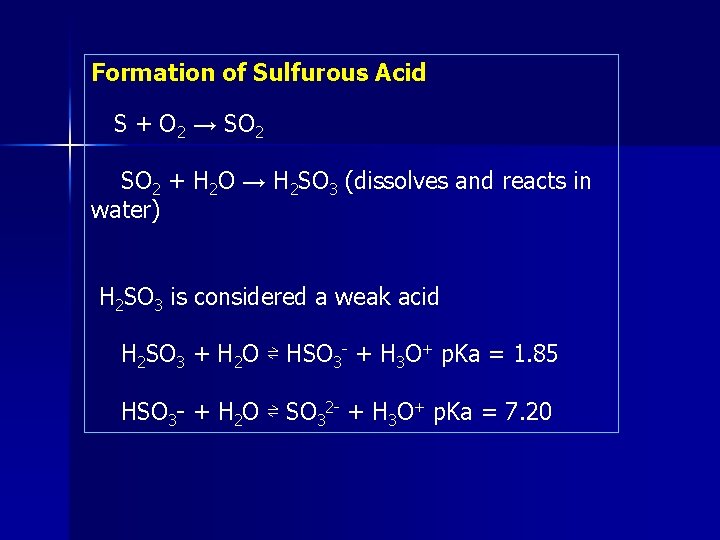
Formation of Sulfurous Acid S + O 2 → SO 2 + H 2 O → H 2 SO 3 (dissolves and reacts in water) H 2 SO 3 is considered a weak acid H 2 SO 3 + H 2 O ⇌ HSO 3 - + H 3 O+ p. Ka = 1. 85 HSO 3 - + H 2 O ⇌ SO 32 - + H 3 O+ p. Ka = 7. 20

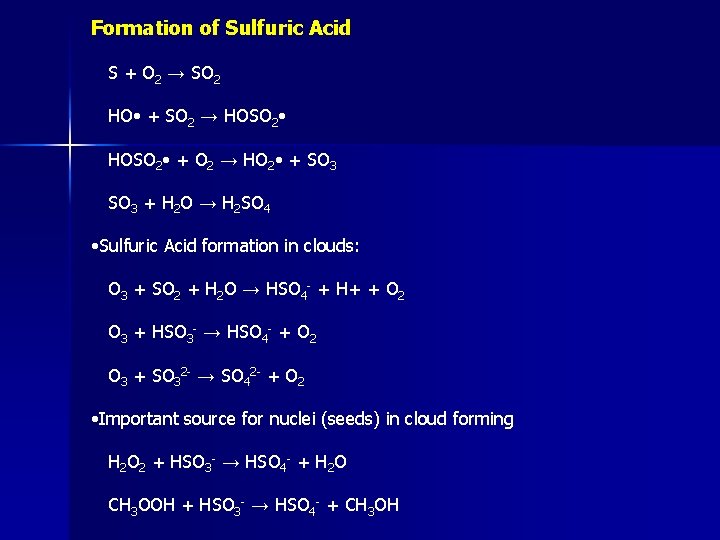
Formation of Sulfuric Acid S + O 2 → SO 2 HO • + SO 2 → HOSO 2 • + O 2 → HO 2 • + SO 3 + H 2 O → H 2 SO 4 • Sulfuric Acid formation in clouds: O 3 + SO 2 + H 2 O → HSO 4 - + H+ + O 2 O 3 + HSO 3 - → HSO 4 - + O 2 O 3 + SO 32 - → SO 42 - + O 2 • Important source for nuclei (seeds) in cloud forming H 2 O 2 + HSO 3 - → HSO 4 - + H 2 O CH 3 OOH + HSO 3 - → HSO 4 - + CH 3 OH

Ammonia In the atmosphere, ammonia neutralizes the acids formed to a large extent, to form ammonium salts. Slightly acidic ammonium salts, (NH 4)2 SO 4 and NH 4 NO 3, formed in the atmosphere sink to the ground or are washed out of the atmosphere with rain. As NH 4+ is deposited and enters the soil, nitrification and acidification can occur. NH 4+ + 2 O 2 → 2 H+ + NO 3 - + H 2 O

Ammonia in the atmosphere can neutralize acid raindrops, forming ammonium salts 2 NH 3 + H 2 SO 4 → (NH 4)2 SO 4 NH 3 + HNO 3 → NH 4 NO 3 • They exist in both aqueous and solid forms. • These salts (formed in atmosphere) are carried to the ground by rain, where they cause damage to plants • They particles also form an aerosol haze in the atmosphere decreasing visibility

Enviro Chemistry Part 12 – Further Water and Soil HL

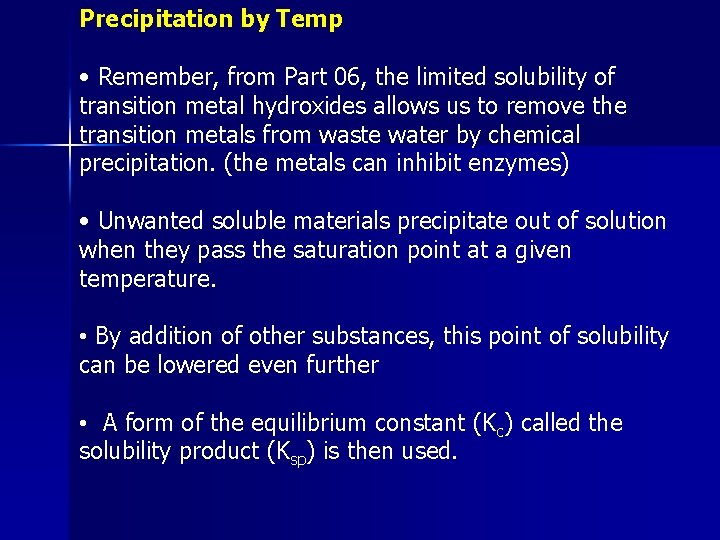
Precipitation by Temp • Remember, from Part 06, the limited solubility of transition metal hydroxides allows us to remove the transition metals from waste water by chemical precipitation. (the metals can inhibit enzymes) • Unwanted soluble materials precipitate out of solution when they pass the saturation point at a given temperature. • By addition of other substances, this point of solubility can be lowered even further • A form of the equilibrium constant (Kc) called the solubility product (Ksp) is then used.

Given the equilibrium formed by a metal M and a non-metal X: MX(s)⇌M+(aq) + X-(aq). • The Keq for this system is given by Ksp = [M+][X−], and is called the solubility product constant.

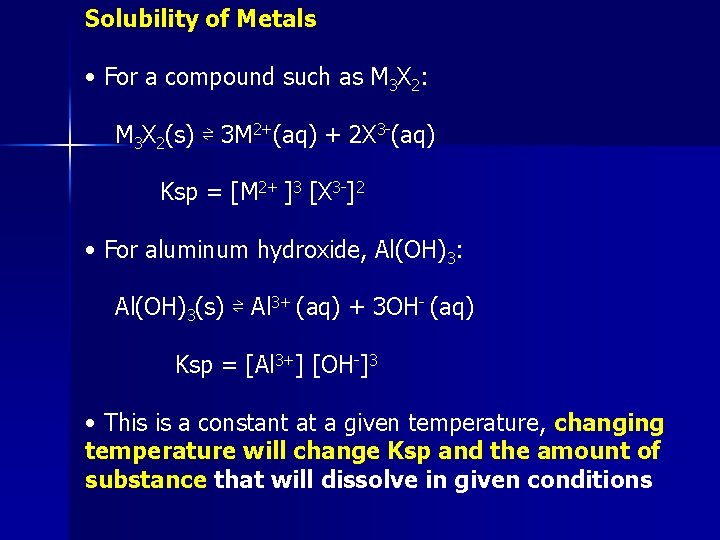
Solubility of Metals • For a compound such as M 3 X 2: M 3 X 2(s) ⇌ 3 M 2+(aq) + 2 X 3 -(aq) Ksp = [M 2+ ]3 [X 3 -]2 • For aluminum hydroxide, Al(OH)3: Al(OH)3(s) ⇌ Al 3+ (aq) + 3 OH- (aq) Ksp = [Al 3+] [OH-]3 • This is a constant at a given temperature, changing temperature will change Ksp and the amount of substance that will dissolve in given conditions

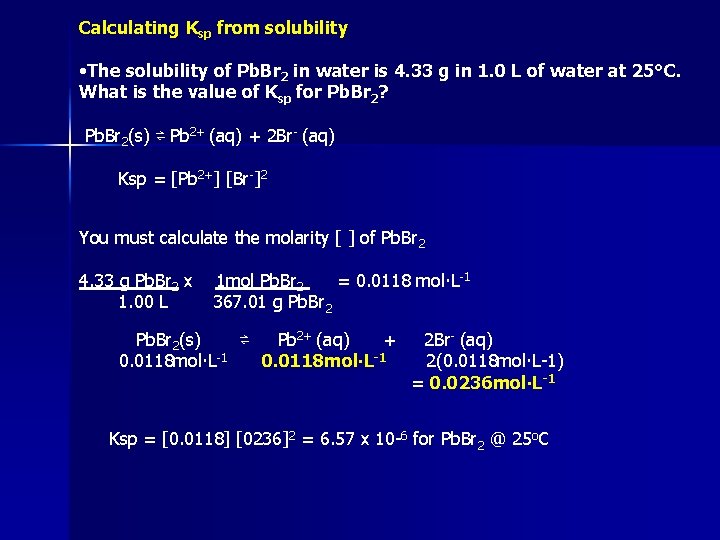
Calculating Ksp from solubility • The solubility of Pb. Br 2 in water is 4. 33 g in 1. 0 L of water at 25°C. What is the value of Ksp for Pb. Br 2? Pb. Br 2(s) ⇌ Pb 2+ (aq) + 2 Br- (aq) Ksp = [Pb 2+] [Br-]2 You must calculate the molarity [ ] of Pb. Br 2 4. 33 g Pb. Br 2 x 1. 00 L 1 mol Pb. Br 2 = 0. 0118 mol∙L-1 367. 01 g Pb. Br 2(s) ⇌ Pb 2+ (aq) + 0. 0118 mol∙L-1 2 Br- (aq) 2(0. 0118 mol∙L-1) = 0. 0236 mol∙L-1 Ksp = [0. 0118] [0236]2 = 6. 57 x 10 -6 for Pb. Br 2 @ 25 o. C

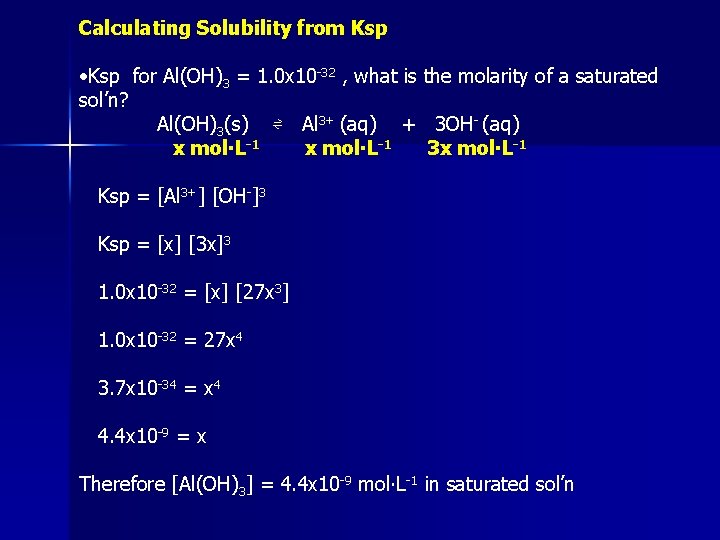
Calculating Solubility from Ksp • Ksp for Al(OH)3 = 1. 0 x 10 -32 , what is the molarity of a saturated sol’n? Al(OH)3(s) ⇌ Al 3+ (aq) + 3 OH- (aq) x mol∙L-1 3 x mol∙L-1 Ksp = [Al 3+] [OH-]3 Ksp = [x] [3 x]3 1. 0 x 10 -32 = [x] [27 x 3] 1. 0 x 10 -32 = 27 x 4 3. 7 x 10 -34 = x 4 4. 4 x 10 -9 = x Therefore [Al(OH)3] = 4. 4 x 10 -9 mol∙L-1 in saturated sol’n

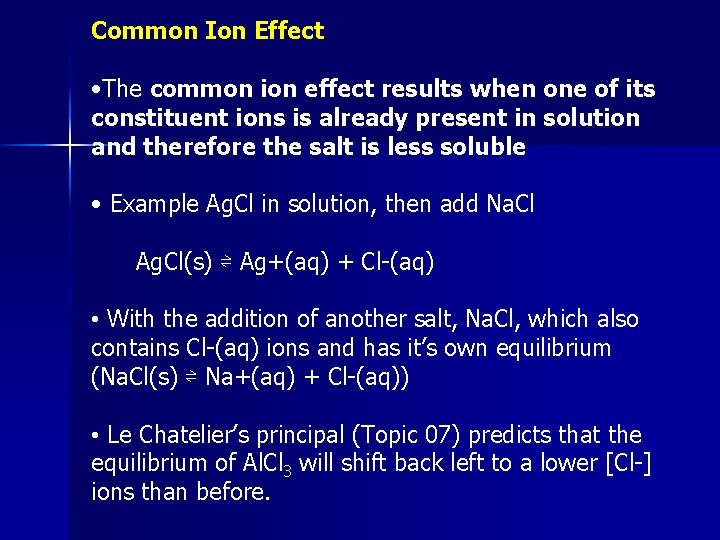
Common Ion Effect • The common ion effect results when one of its constituent ions is already present in solution and therefore the salt is less soluble • Example Ag. Cl in solution, then add Na. Cl Ag. Cl(s) ⇌ Ag+(aq) + Cl-(aq) • With the addition of another salt, Na. Cl, which also contains Cl-(aq) ions and has it’s own equilibrium (Na. Cl(s) ⇌ Na+(aq) + Cl-(aq)) • Le Chatelier’s principal (Topic 07) predicts that the equilibrium of Al. Cl 3 will shift back left to a lower [Cl-] ions than before.

Ag. Cl in Water • Consider the following example: • For Ag. Cl Ksp=2. 0 x 10 -10 at 298 K, dissolved in H 2 O Ag. Cl(s) ⇌ Ag+(aq) + Cl-(aq) Ksp = [Ag+(aq)][Cl-(aq)] (where they are equal, and “x”) Ksp = x 2 = 2. 0 x 10 -10 x = (2. 0 x 10 -10)1/2 x = 1. 4 x 10 -5 mol/dm 3 (molarity of Ag. Cl in water)

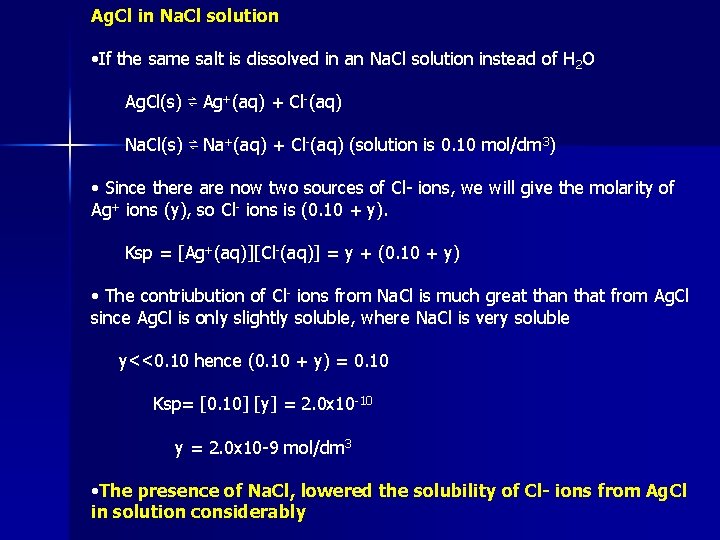
Ag. Cl in Na. Cl solution • If the same salt is dissolved in an Na. Cl solution instead of H 2 O Ag. Cl(s) ⇌ Ag+(aq) + Cl-(aq) Na. Cl(s) ⇌ Na+(aq) + Cl-(aq) (solution is 0. 10 mol/dm 3) • Since there are now two sources of Cl- ions, we will give the molarity of Ag+ ions (y), so Cl- ions is (0. 10 + y). Ksp = [Ag+(aq)][Cl-(aq)] = y + (0. 10 + y) • The contriubution of Cl- ions from Na. Cl is much great than that from Ag. Cl since Ag. Cl is only slightly soluble, where Na. Cl is very soluble y<<0. 10 hence (0. 10 + y) = 0. 10 Ksp= [0. 10] [y] = 2. 0 x 10 -10 y = 2. 0 x 10 -9 mol/dm 3 • The presence of Na. Cl, lowered the solubility of Cl- ions from Ag. Cl in solution considerably

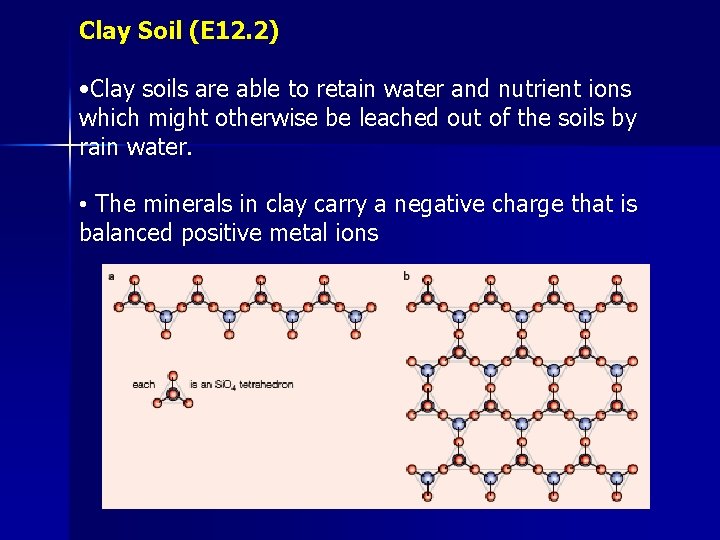
Clay Soil (E 12. 2) • Clay soils are able to retain water and nutrient ions which might otherwise be leached out of the soils by rain water. • The minerals in clay carry a negative charge that is balanced positive metal ions

Cation-exchange capacity (CEC) • The cations bound to the clay structure are not permanent as they can be exchanged. Clay-−Na+(s) + H+(aq) ⇌ Clay-−H+(s) + Na+(aq) • Cation-exchange capacity (CEC) is defined as the amount of the single-positive cations that can be exchanged with the soil solution, per Kg of clay • If the clay contains Mg 2+ or Al 3+, the replacement of these ions increases the negative charge of the soil and CEC increases. • CEC values range from 0. 03 for kaolinite up to 1. 5 for vermiculite clay. • Most soils only contain small fraction of clay, so CEC’s for soils range from 0. 02 – 0. 60

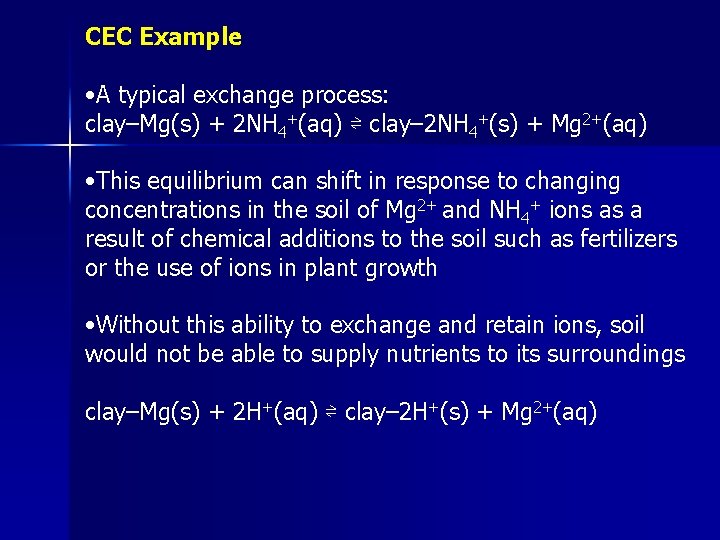
CEC Example • A typical exchange process: clay–Mg(s) + 2 NH 4+(aq) ⇌ clay– 2 NH 4+(s) + Mg 2+(aq) • This equilibrium can shift in response to changing concentrations in the soil of Mg 2+ and NH 4+ ions as a result of chemical additions to the soil such as fertilizers or the use of ions in plant growth • Without this ability to exchange and retain ions, soil would not be able to supply nutrients to its surroundings clay–Mg(s) + 2 H+(aq) ⇌ clay– 2 H+(s) + Mg 2+(aq)

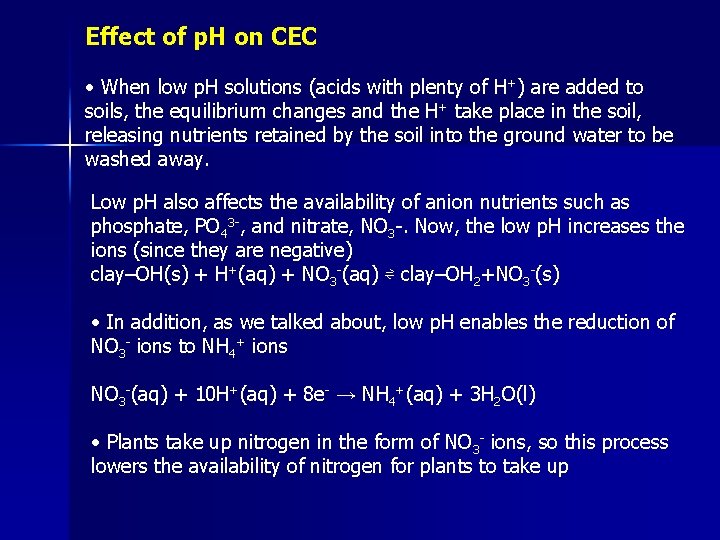
Effect of p. H on CEC • When low p. H solutions (acids with plenty of H+) are added to soils, the equilibrium changes and the H+ take place in the soil, releasing nutrients retained by the soil into the ground water to be washed away. Low p. H also affects the availability of anion nutrients such as phosphate, PO 43 -, and nitrate, NO 3 -. Now, the low p. H increases the ions (since they are negative) clay–OH(s) + H+(aq) + NO 3 -(aq) ⇌ clay–OH 2+NO 3 -(s) • In addition, as we talked about, low p. H enables the reduction of NO 3 - ions to NH 4+ ions NO 3 -(aq) + 10 H+(aq) + 8 e- → NH 4+(aq) + 3 H 2 O(l) • Plants take up nitrogen in the form of NO 3 - ions, so this process lowers the availability of nitrogen for plants to take up

Read p. 722 -724 Brown-Ford for more equations involving soil p. H on CEC and availability of nutrients.

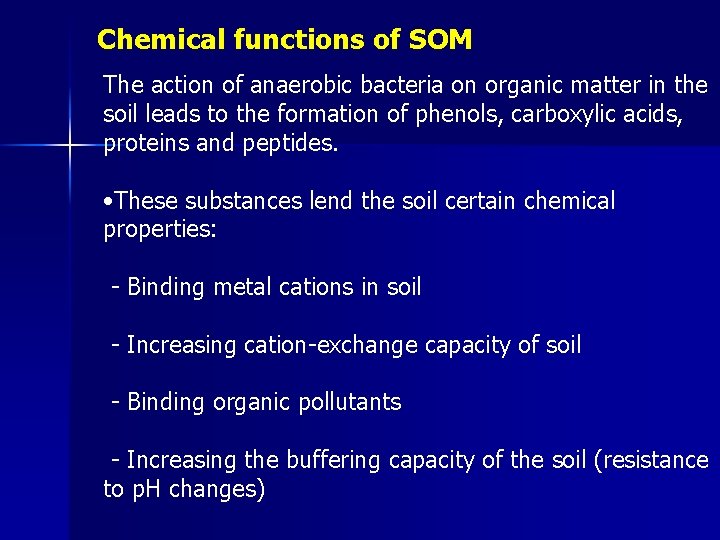
Chemical functions of SOM The action of anaerobic bacteria on organic matter in the soil leads to the formation of phenols, carboxylic acids, proteins and peptides. • These substances lend the soil certain chemical properties: - Binding metal cations in soil - Increasing cation-exchange capacity of soil - Binding organic pollutants - Increasing the buffering capacity of the soil (resistance to p. H changes)

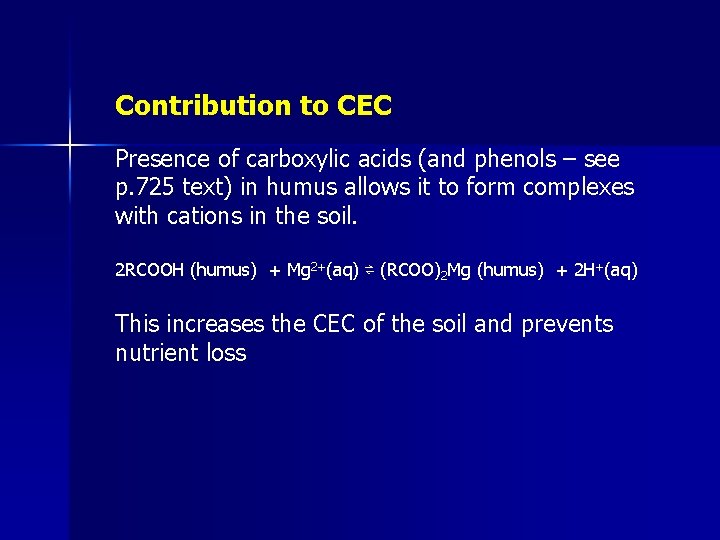
Contribution to CEC Presence of carboxylic acids (and phenols – see p. 725 text) in humus allows it to form complexes with cations in the soil. 2 RCOOH (humus) + Mg 2+(aq) ⇌ (RCOO)2 Mg (humus) + 2 H+(aq) This increases the CEC of the soil and prevents nutrient loss

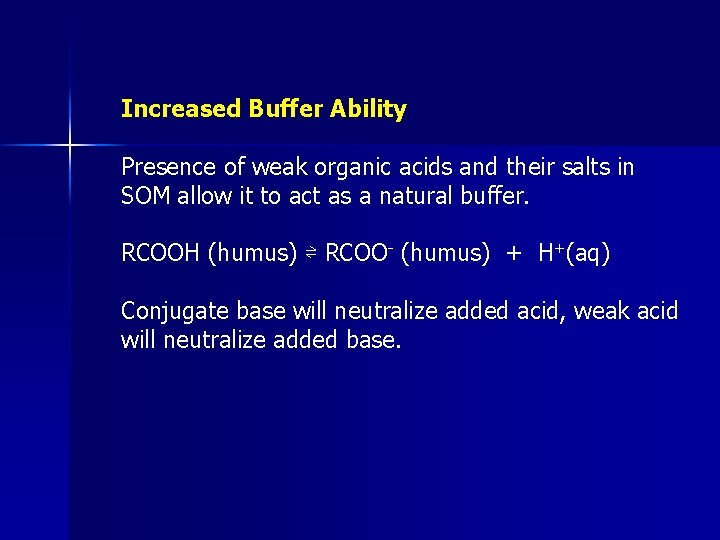
Increased Buffer Ability Presence of weak organic acids and their salts in SOM allow it to act as a natural buffer. RCOOH (humus) ⇌ RCOO- (humus) + H+(aq) Conjugate base will neutralize added acid, weak acid will neutralize added base.

Other functions of SOM As well as binding to nutrient cations, SOM is able to form complexes with toxic aluminum and heavy-metal cations preventing them from entering sol’n. • • As humic substances are organic in nature, they are able to absorb other organic substances such as herbicides and pesticides , decreasing pollution reaching and affecting water supply

IB happy ‘cause IB DONE