Environmental Assessment of Genetically Engineered Animals at CVM










- Slides: 16

Environmental Assessment of Genetically Engineered Animals at CVM (FDA) Animal Biotechnology Interdisciplinary Group Center for Veterinary Medicine U. S. Food and Drug Administration Evgenij A. Evdokimov, Ph. D Erik M. Silberhorn, Ph. D

Major Statutes Governing Regulation of GE Animals Federal Food, Drug, and Cosmetic Act (FD&C Act) ¢ Products are regulated; not processes National Environmental Policy Act (NEPA) ¢ Procedural; orders agencies to evaluate impacts of “agency actions”

Guidance for Industry 187* ¢ ¢ Covers all types of GE animals Definition of “article” l ¢ ¢ ¢ r. DNA construct intended to affect the structure or function of the animal All GE animals in a lineage are covered Event-based, case-by-case evaluation Enforcement discretion and approval paths New Animal Drug Application (NADA) means mandatory approval prior to marketing Post-market surveillance *http: //www. fda. gov/Animal. Veterinary/Development. Approval. Process/Genetic. Engineering/Genetically. Engine ered. Animals/default. htm

Statutory/Regulatory Requirements ¢ Sponsor must submit Environmental Assessment/supporting data under INAD/NADA ¢ National Environmental Policy Act (NEPA) requirement triggered by “agency action” l EA FONSI? (finding of no significant impact) l If no FONSI, EIS (environmental impact statement)

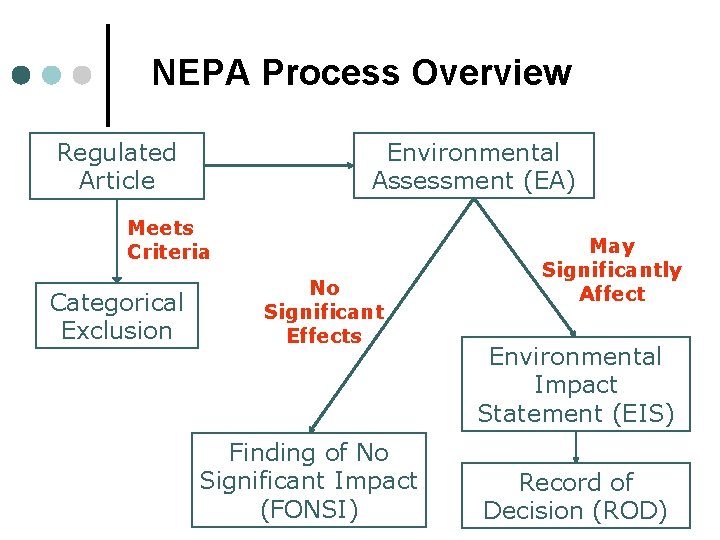
NEPA Process Overview Regulated Article Environmental Assessment (EA) Meets Criteria Categorical Exclusion No Significant Effects Finding of No Significant Impact (FONSI) May Significantly Affect Environmental Impact Statement (EIS) Record of Decision (ROD)

Hierarchical Risk-Based Evaluation Are there significant direct or indirect effects from introduction of the GE animal into the environment? Basis for satisfying NEPA requirements.

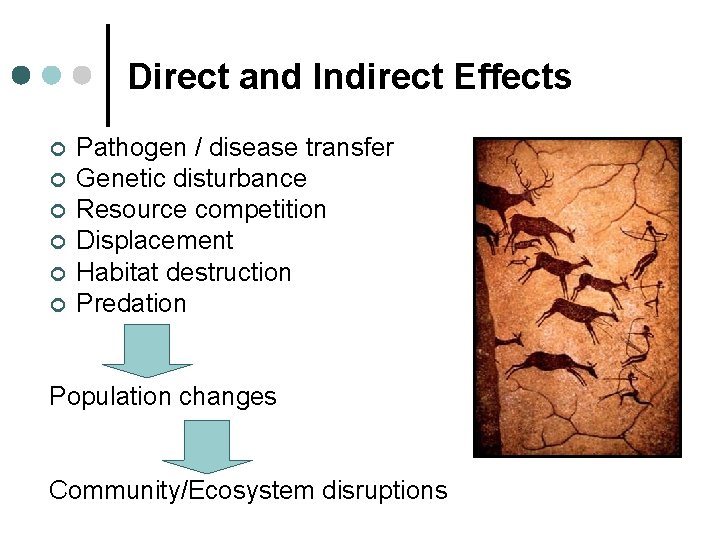
Direct and Indirect Effects ¢ ¢ ¢ Pathogen / disease transfer Genetic disturbance Resource competition Displacement Habitat destruction Predation Population changes Community/Ecosystem disruptions

Environmental Assessment: General Risk Questions For a specific GE animal (population) containing a specific r. DNA construct…. l Likelihood of escape? • Containment/redundancy l l l Likelihood of survival if escape occurs? Likelihood of establishment and reproduction? Potential consequences/effects associated with escape? Considered in context of appropriate comparator on a case-by-case basis

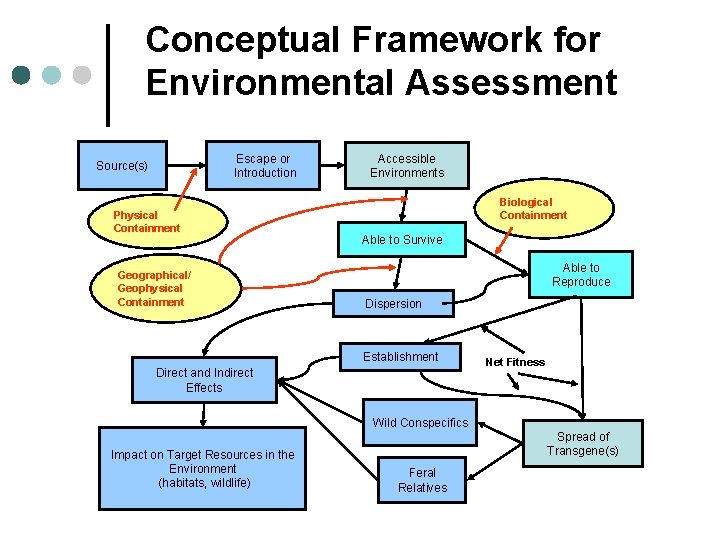
Conceptual Framework for Environmental Assessment Escape or Introduction Source(s) Physical Containment Geographical/ Geophysical Containment Accessible Environments Biological Containment Able to Survive Able to Reproduce Dispersion Establishment Direct and Indirect Effects Net Fitness Wild Conspecifics Impact on Target Resources in the Environment (habitats, wildlife) Spread of Transgene(s) Feral Relatives

Consequences of Introduction, Escape, and Dispersion Will depend largely on: ¢ Specific physical locations of production or release ¢ Extent of containment (if applicable) l l ¢ ¢ Physical/mechanical Biological (e. g. , sterility, monosex) Geographical/geophysical (environmental conditions) Domestication of species (ability to become feral) Mobility of species “Net fitness”

Fitness* ¢ ¢ ¢ Genetic contribution by an individual’s descendants to future generations of a population Fitness depends on both survival and reproduction Net fitness components include l Juvenile and adult viability l Age at sexual maturity l Female fecundity/male fertility/mating success * These characteristics are used to assess fitness regardless of an animal’s GE status

Definitions, Relationships, Standards Harm ≡ an adverse outcome Hazard ≡ substance or activity that has the potential to cause a harm Risk ≡ conditional probability of an adverse outcome provided that exposure to a receptor has occurred …or Risk foutcome (exposure, hazard), or “the likelihood of harm” Receptor ≡ individual or population experiencing risk Safety …. reasonable certainty of no harm (established standard)

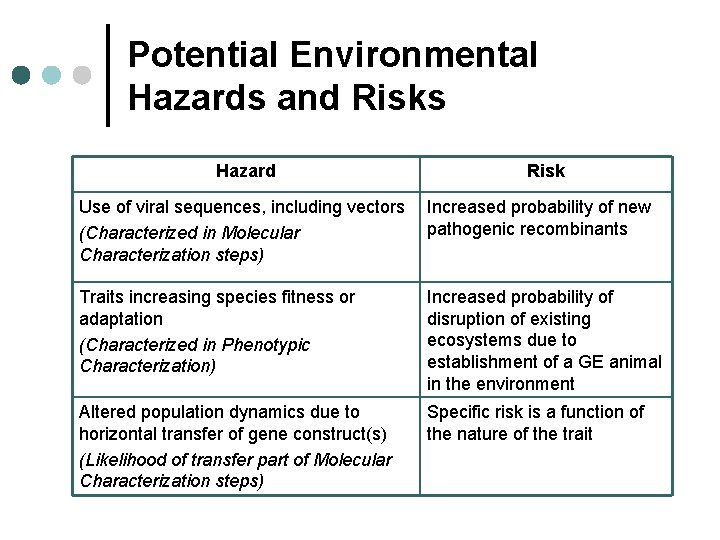
Potential Environmental Hazards and Risks Hazard Risk Use of viral sequences, including vectors (Characterized in Molecular Characterization steps) Increased probability of new pathogenic recombinants Traits increasing species fitness or adaptation (Characterized in Phenotypic Characterization) Increased probability of disruption of existing ecosystems due to establishment of a GE animal in the environment Altered population dynamics due to horizontal transfer of gene construct(s) (Likelihood of transfer part of Molecular Characterization steps) Specific risk is a function of the nature of the trait

Areas of interest ¢ Containment effectiveness and ways to ensure containment of GE animals l l l ¢ ¢ Physical/mechanical Biological (e. g. , sterility, monosex) Geographical/geophysical (environmental conditions) Fitness Case by case evaluation of GE animals

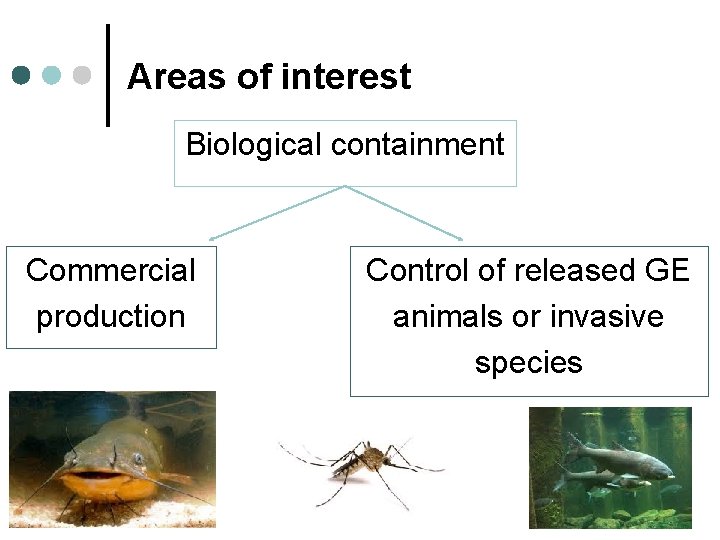
Areas of interest Biological containment Commercial production Control of released GE animals or invasive species

Questions? Contacts at CVM: Dr. Eric Silberhorn eric. silberhorn@fda. hhs. gov Dr. Evgenij Evdokimov evgenij. evdokimov@fda. hhs. gov
 Contingent valuation method (cvm)
Contingent valuation method (cvm) Caixa cvm ited dimensões
Caixa cvm ited dimensões Crowdfunding cvm
Crowdfunding cvm Bruno gomes cvm
Bruno gomes cvm Cvm investors hub
Cvm investors hub Customer visit management
Customer visit management Customer value management in banking
Customer value management in banking Genetically modified organisms
Genetically modified organisms Genetically modified organisms
Genetically modified organisms Chapter 22 genetics and genetically linked diseases
Chapter 22 genetics and genetically linked diseases Genetically modified crops
Genetically modified crops Biotechnology examples
Biotechnology examples Are fingerprint patterns inherited
Are fingerprint patterns inherited Genetically modified crops have
Genetically modified crops have Genetically modified crops have
Genetically modified crops have Genetically modified crops have
Genetically modified crops have Diverse offspring
Diverse offspring