Enveloped RNA Viruses Paramyxoviruses Lecture 4 Paramyxoviruses Measles





















- Slides: 45

Enveloped RNA Viruses Paramyxoviruses Lecture 4


Paramyxoviruses Measles, mumps, parainfluenza viruses, respiratory syncytial virus, human metapneumovirus


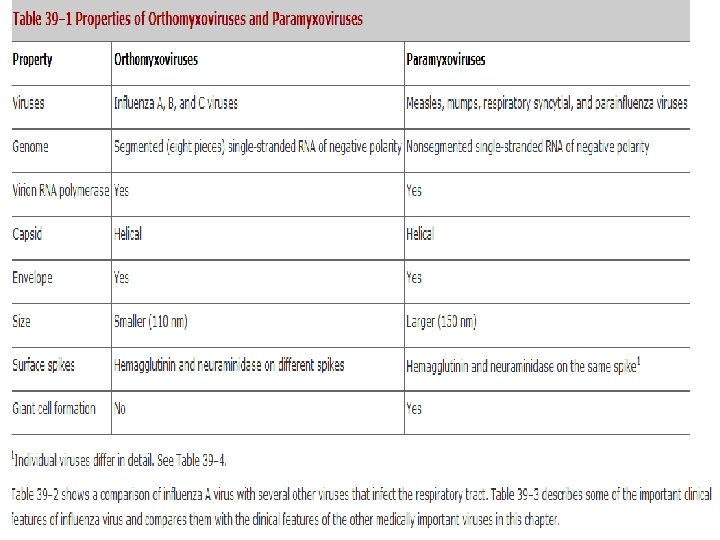
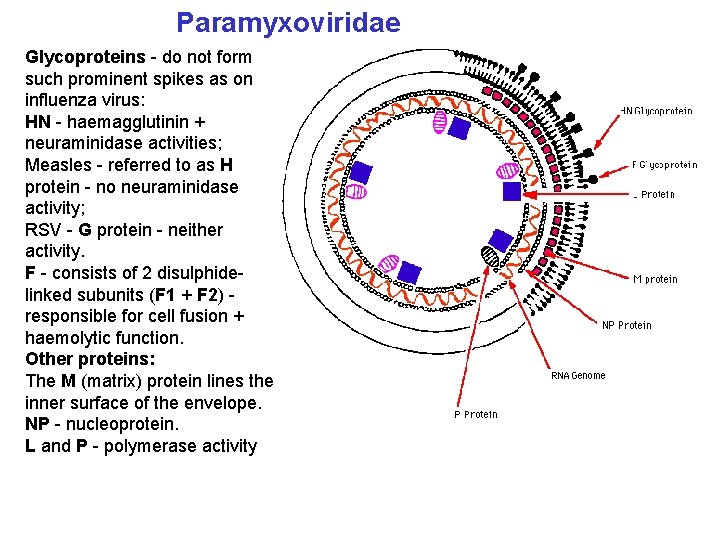
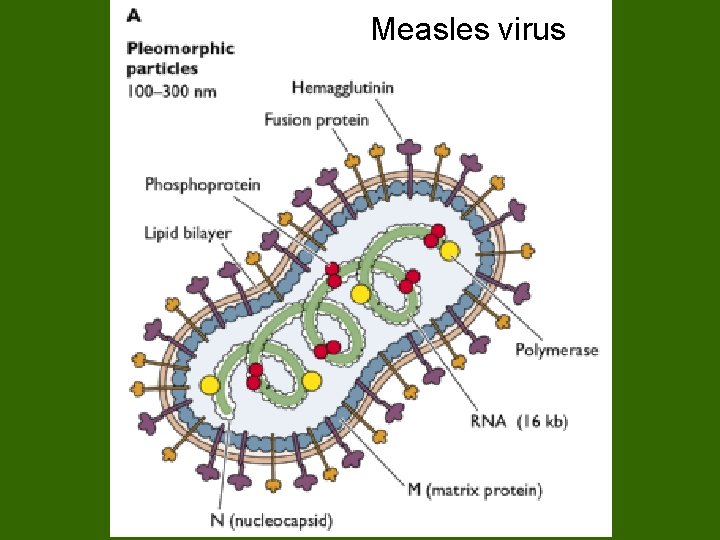
Paramyxoviridae Glycoproteins - do not form such prominent spikes as on influenza virus: HN - haemagglutinin + neuraminidase activities; Measles - referred to as H protein - no neuraminidase activity; RSV - G protein - neither activity. F - consists of 2 disulphidelinked subunits (F 1 + F 2) responsible for cell fusion + haemolytic function. Other proteins: The M (matrix) protein lines the inner surface of the envelope. NP - nucleoprotein. L and P - polymerase activity

Paramyxovirus structure Paramyxovirus electron micrograph

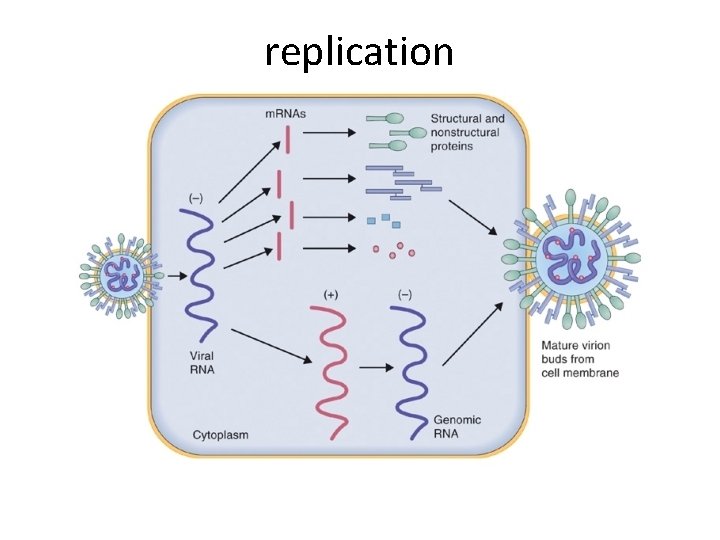
replication

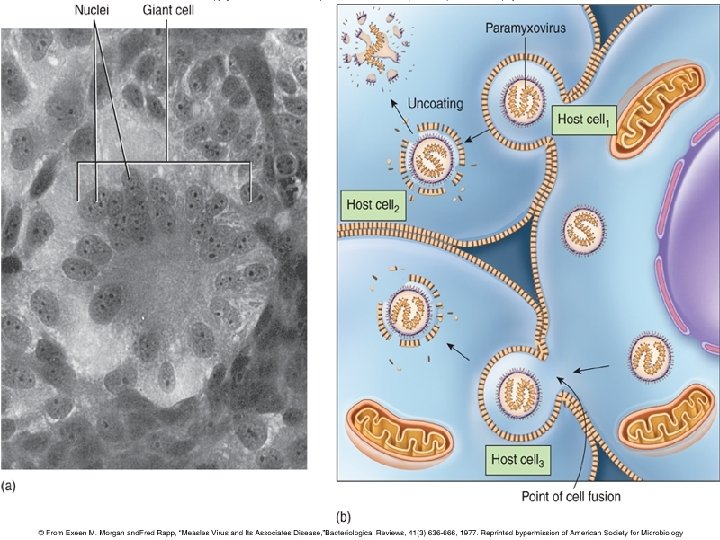
Paramyxoviruses • Envelope has HN and specialized F spikes that initiate cell-to-cell fusion. • Fusion with neighboring cells – syncytium or multinucleate giant cells form 8

Insert figure 25. 5 Effects of paramyxoviruses 9

Measles: general • One of the five classical childhood exanthems (eruptive diseases) – rubella (togavirus) – roseola (HHV 6) – fifth disease (parvovirus, B 19, erythema infectiosum) – chickenpox (herpes zoster) • Host range limited to humans, one serotype

Measles virus

Measles • • Caused by Morbillivirus Also known as red measles and rubeola Different from German measles Very contagious; transmitted by respiratory aerosols • Humans are the only reservoir. 12

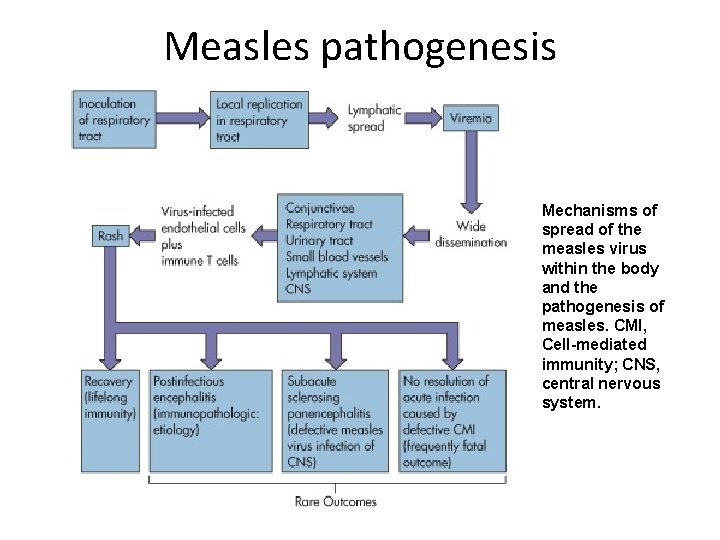
Measles pathogenesis Mechanisms of spread of the measles virus within the body and the pathogenesis of measles. CMI, Cell-mediated immunity; CNS, central nervous system.

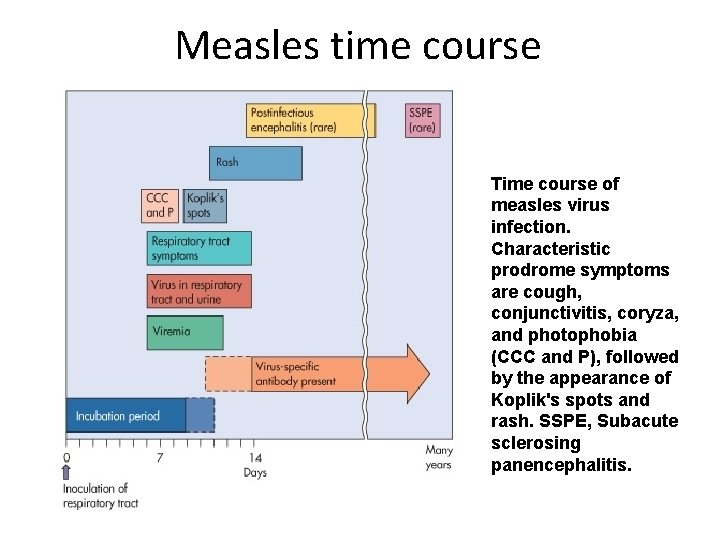
Measles time course Time course of measles virus infection. Characteristic prodrome symptoms are cough, conjunctivitis, coryza, and photophobia (CCC and P), followed by the appearance of Koplik's spots and rash. SSPE, Subacute sclerosing panencephalitis.

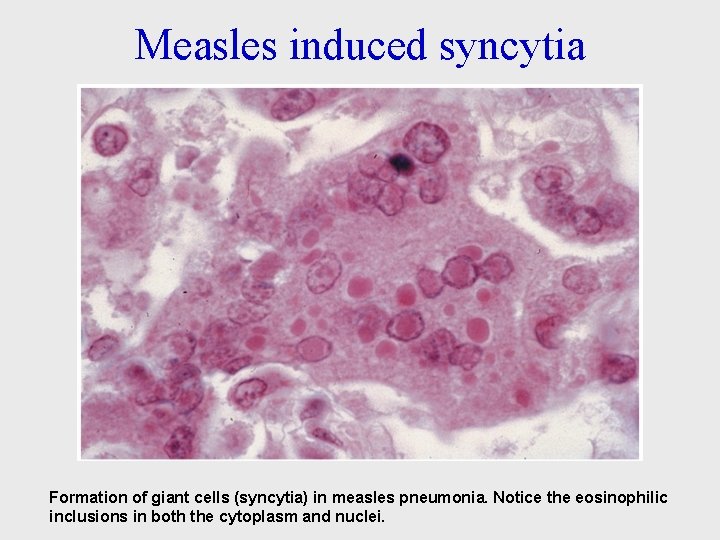
Measles induced syncytia Formation of giant cells (syncytia) in measles pneumonia. Notice the eosinophilic inclusions in both the cytoplasm and nuclei.

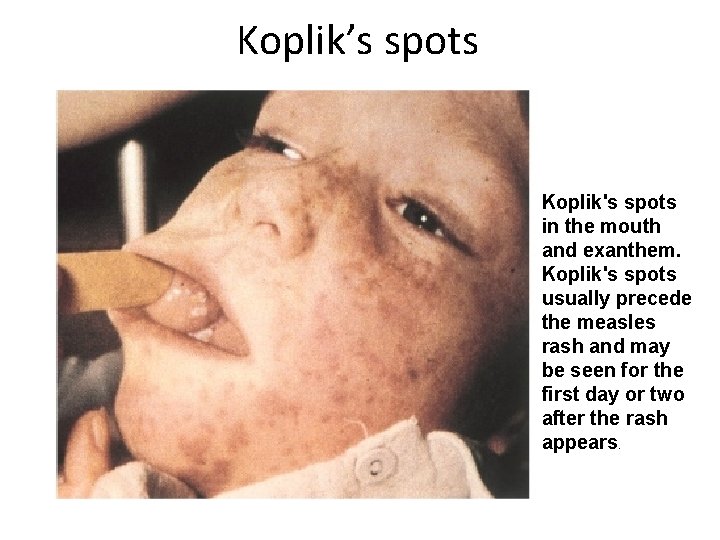
Koplik’s spots Koplik's spots in the mouth and exanthem. Koplik's spots usually precede the measles rash and may be seen for the first day or two after the rash appears.

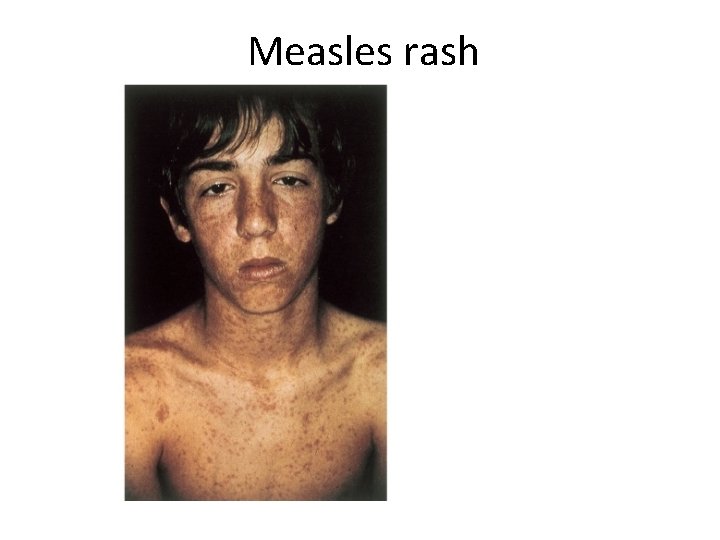
Measles rash

Measles • Symptoms start about 10 days after exposure – Average 10 days from exposure to onset of fever – Average 14 days from exposure to onset of rash • Other symptoms and complications – Ear infection – Pneumonia – CNS/ brain infection (as SSPE, subacute sclerosing panencephalitis) – Complications may be lethal – More serious in infants and adults, less in children and teens • Vaccine – Measles (Paramyxoviridae), mumps (Paramyxoviridae), rubella (Togaviridae, + sense) (MMR) vaccine is a live vaccine – Has been very effective in limiting spread – Links of vaccine to autism have been proposed but not shown

Subacute-sclerosing panencephalitis (SSPE) • Most serious complication is subacute sclerosing panencephalitis (SSPE), a progressive neurological degeneration of the cerebral cortex, white matter and brain stem. • Neural manifestation of measles virus • Defective form spreading through the brain by cell fusion and destroys cells • Specific mutations result in virus that is more fit in neural cells • Usually occurs 6 -12 years after measles infection • More rarely associated with live measles vaccine • Infection rate 1 per million; higher in males & rural • Mortality high leads to coma and death in months or year

Mumps

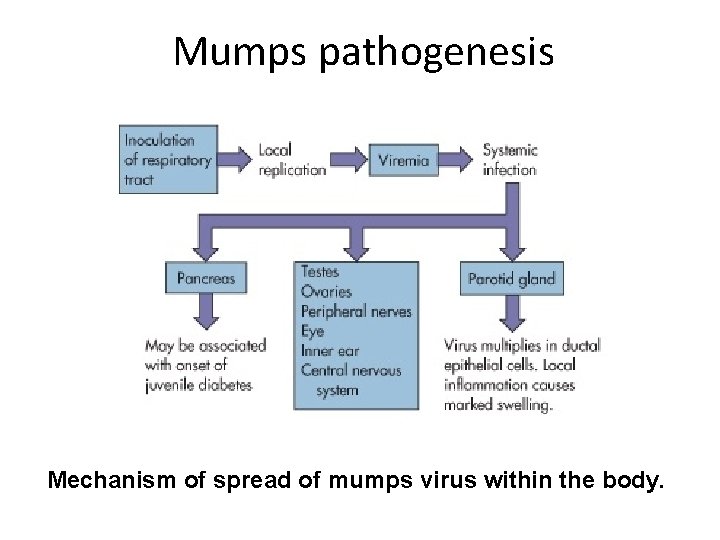
Mumps pathogenesis Mechanism of spread of mumps virus within the body.

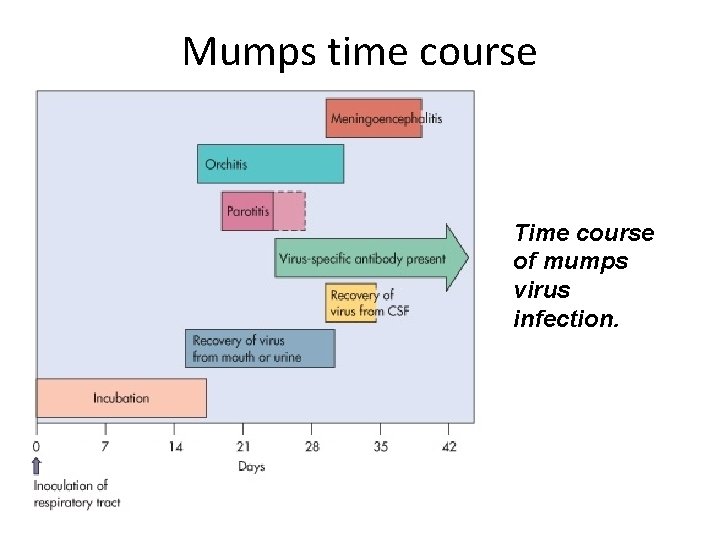
Mumps time course Time course of mumps virus infection.

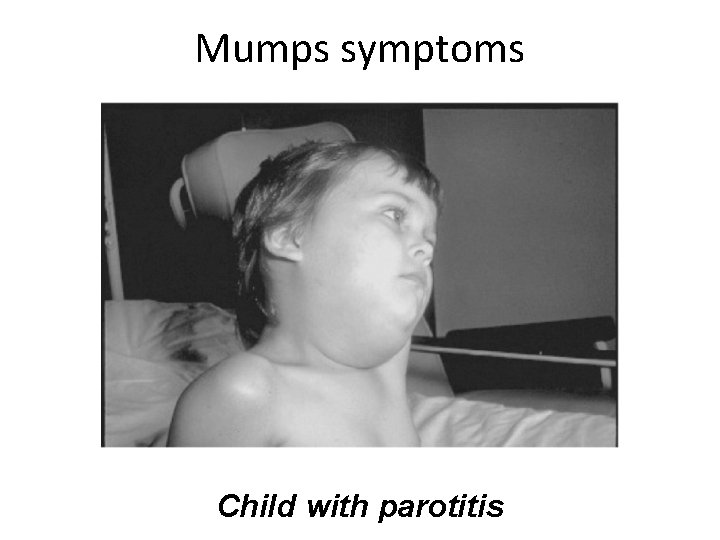
Mumps symptoms Child with parotitis

Mumps • Epidemic parotitis; self-limited, associated with painful swelling of parotid salivary glands • Humans are the only reservoir. • 40% of infections are subclinical; long-term immunity. • Incubation 2 -3 weeks fever, muscle pain and malaise, classic swelling of one or both cheeks • Usually uncomplicated invasion of other organs; in 20 -30% of infected adult males, epididymis and testes become infected; sterility is rare • Symptomatic treatment • Live attenuated vaccine MMR 24

Laboratory Diagnosis The diagnosis of mumps is usually made clinically, but laboratory tests are available for confirmation. The virus can be isolated in cell culture from saliva, spinal fluid, or urine. In addition, a fourfold rise in antibody titer in either the hemagglutination inhibition or the CF test is diagnostic. A single CF test that assays both the S and the V (viral) antigens can also be used. Because antibody to S antigen appears early and is short-lived, it indicates current infection. If only V antibody is found, the patient has had mumps in the past. A mumps skin test based on delayed hypersensitivity can be used to detect previous infection, but serologic tests are preferred. The mumps skin test is widely used to determine whether a patient's cell-mediated immunity is competent. 25

In the late 1980 s, outbreaks of mumps occurred in both immunized and unimmunized people. This led to the recommendation in 1989 that a second course of MMR (measles, mumps, rubella) vaccine be administered. The incidence of mumps fell and outbreaks did not occur until 2006 when 6584 cases occurred, primarily in college-age individuals who, surprisingly, had received two doses of the vaccine. Waning immunity after the second dose and immunization with a different genotype from the genotype that caused the outbreak are 26 suggested explanations.

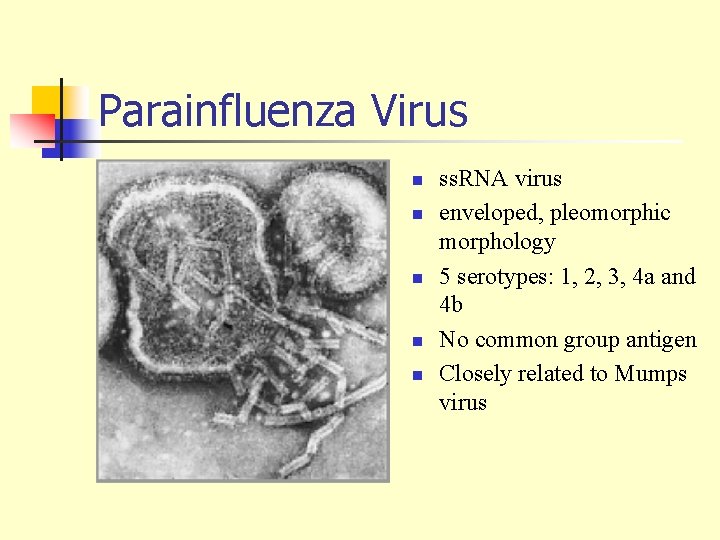
Parainfluenza Virus n n n ss. RNA virus enveloped, pleomorphic morphology 5 serotypes: 1, 2, 3, 4 a and 4 b No common group antigen Closely related to Mumps virus

Parainfluenza viruses • Four serotypes, host range limited to humans, • Respiratory transmission • Infections limited to respiratory tract, generally non-systemic and viremia rare • Causes cold-like symptoms, bronchitis and croup (laryngotraheobroncitis) (serotypes 1 and 2), Bronchiolitis, . Infections of children common • Diagnosis: Detection of Antigen - from nasopharyngeal aspirates and throat washings. – Virus culture – Syncytia formation – Hemadsorption – Hemagglutination inhibition – rt. PCR – Serology - a retrospective diagnosis may be made by serology. CFT most widely used • Immunity following infection short-lived. Individuals subject to reinfection

Management • No specific antiviral chemotherapy available. • Severe cases of croup should be admitted to hospital and placed in oxygen tents. • No vaccine is available.

Respiratory Syncytial Virus (RSV) n n n ss. RNA eveloped virus. belong to the genus Pneumovirus of the Paramyxovirus family. Considerable strain variation exists, may be classified into subgroups A and B by monoclonal sera. n Both subgroups circulate in the community at any one time. n Causes a sizable epidemic each year.

Clinical Manifestations n n Most common cause of severe lower respiratory tract disease in infants, responsible for 50 -90% of Bronchiolitis and 5 -40% of Bronchopneumonia Other manifestations include croup (10% of all cases). Infants at Risk of Severe Infection 1. Infants with congenital heart disease 2. Infants with underlying pulmonary disease 3. Immunocompromized infants In older children and adults, the symptoms are much milder: it may cause a corza-like illness or bronchitis.

Respiratory syncytial virus Widespread: 75% of infants seropositive by 1 year of age Host range limited to humans; single serotype Respiratory transmission – Highly contagious; contagion period precedes symptoms and may occur in absence of symptoms • Localized infections of respiratory tract, no viremia and no systemic infections q Poor immunity – Reinfection occurs throughout life – Maternal antibody does not prevent infection v Diagnosis; Detection of Antigen - a rapid diagnosis can be made by the detection of RSV antigen from nasopharyngeal aspirates. A rapid diagnosis is important because of the availability of therapy Virus Isolation - virus may be readily isolated from nasopharyngeal aspirates. However, this will take several days. rt. PCR, immunofluorescence, enzyme immunoassay, Serology; Serology - a retrospective diagnosis may be made by serology. CFT most widely used. • • •

Treatment and Prevention Treatment n Ribavarin reduces severity of symptoms in immunocomprimized patients. Aerosolised ribavirin can be used for infants with severe infection, and for those at risk of severe disease No vaccine; improper vaccination increases severity of disease n Passive vaccination for high risk infants n Palivizumab: anti-F monoclonal antibody. RSV immunoglobulin can be used to protect infants at risk of severe RSV disease. v

Rubella

Important Properties Rubella virus is a member of the togavirus family. It is composed of one piece of single-stranded RNA, an icosahedral nucleocapsid, and a lipoprotein envelope. It has a positivestrand RNA and therefore has no virion polymerase. Its surface spikes contain hemagglutinin. The virus has a single antigenic type. Antibody against hemagglutinin neutralizes infectivity. Humans are the natural host.

Rubella n n From Latin meaning "little red" Discovered in 18 th century - thought to be variant of measles First described as distinct clinical entity in German literature Congenital rubella syndrome (CRS) described by Gregg in 1941

Rubella • Caused by Rubivirus, a Togavirus • ss. RNA with a loose envelope • Endemic disease. The disease occurs worldwide. In areas where the vaccine is not used, epidemics occur every 6 to 9 years. : • Most cases reported are adolescents and young adults. Two clinical forms: • Postnatal rubella – malaise, fever, sore throat, lymphadenopathy, rash, generally mild, lasting about 3 days • Congenital rubella – infection during 1 st trimester 37

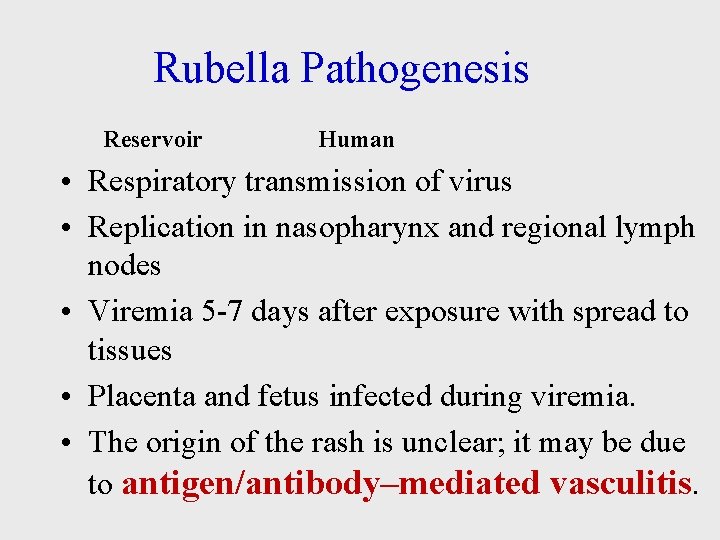
Rubella Pathogenesis Reservoir Human • Respiratory transmission of virus • Replication in nasopharynx and regional lymph nodes • Viremia 5 -7 days after exposure with spread to tissues • Placenta and fetus infected during viremia. • The origin of the rash is unclear; it may be due to antigen/antibody–mediated vasculitis.

Clinical Findings Rubella is a milder, shorter disease than measles. After an incubation period of 14 to 21 days, a brief prodromal period with fever and malaise is followed by a maculopapular rash, which starts on the face and progresses downward to involve the extremities. Posterior auricular lymphadenopathy is characteristic. The rash typically lasts 3 days. When rubella occurs in adults, especially women, polyarthritis caused by immune complexes often occurs. 39

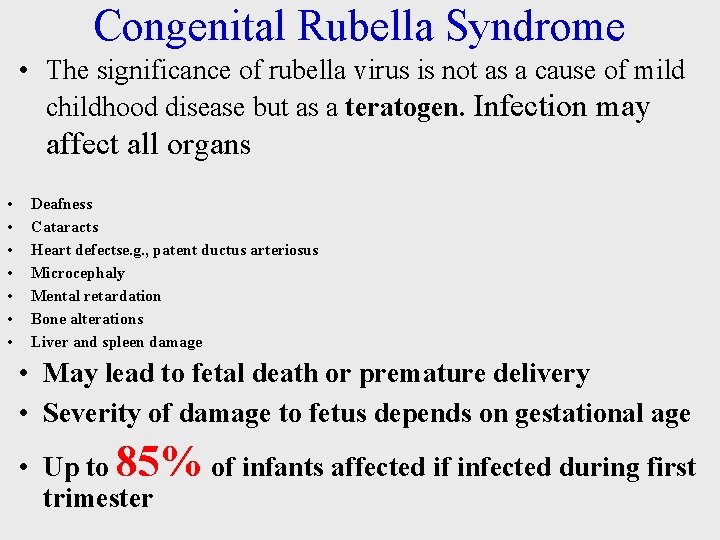
Congenital Rubella Syndrome • The significance of rubella virus is not as a cause of mild childhood disease but as a teratogen. Infection may affect all organs • • Deafness Cataracts Heart defectse. g. , patent ductus arteriosus Microcephaly Mental retardation Bone alterations Liver and spleen damage • May lead to fetal death or premature delivery • Severity of damage to fetus depends on gestational age • Up to 85% of infants affected if infected during first trimester

In addition, some children infected in utero can continue to excrete rubella virus for months following birth, which is a significant public health hazard because the virus can be transmitted to pregnant women. Some congenital shedders are asymptomatic and without malformations and hence can be diagnosed only if the virus is isolated. Congenitally infected infants also have significant Ig. M titers and persistent Ig. G titers long after maternal antibody has disappeared. 41

Rubella Laboratory Diagnosis • Isolation of rubella virus from clinical specimen (e. g. , nasopharynx, urine) no CPE ( interference). • Positive serologic test for rubella Ig. M antibody • Significant rise in rubella Ig. G by any standard serologic assay (e. g. , enzyme immunoassay) If recent infection has occurred, an amniocentesis can reveal whethere is rubella virus in the amniotic fluid, which indicates definite fetal infection. .

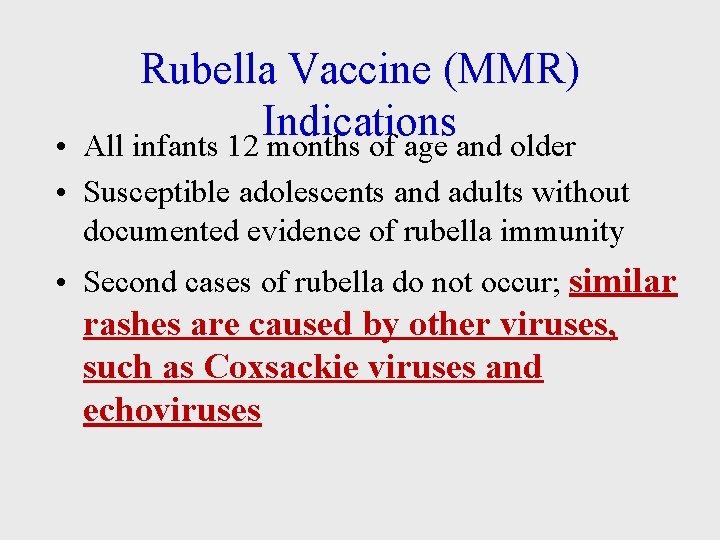
Rubella Vaccine (MMR) Indications All infants 12 months of age and older • • Susceptible adolescents and adults without documented evidence of rubella immunity • Second cases of rubella do not occur; similar rashes are caused by other viruses, such as Coxsackie viruses and echoviruses

Immune serum globulins (IG) can be given to pregnant women in the first trimester, who have been exposed to a known case of rubella and for whom termination of the pregnancy is not an option. The main problems with giving IG are that there are instances in which it fails to prevent fetal infection and that it may confuse the interpretation of serologic tests. If termination of the pregnancy is an option, it is recommended to attempt to determine whether the mother and fetus have been infected 44

get well after a period of sickness to be immune for the rest of their lives. Examples are MEASLES INFECTION, RUBELLA or German measles, MUMPS and many others. . .