ENVE 201 Environmental Engineering Chemistry 1 COLOR Chapter












- Slides: 15

ENVE 201 Environmental Engineering Chemistry 1 COLOR (Chapter 13) Dr. Aslıhan Kerç

Color • Most of the surface water are colored, especially in swampy areas. Coloring material due to contact of water with organic debris (decomposition of leaves, woods)

Principal Color Bodies • Tannins • Humic Acid • Humates If iron is also present Ferric humates

• Natural color Negatively charged colloidal particles. can be removed by coagulation Surface waters may appear highly colored due to colored suspended matter (red clay soil)

Types of Color • Apparent Color: Color caused by suspended matter • True color: Color due to vegetable or organic extracts that are colloidal.

• Color intensity ↗ with p. H ↗ It is important to record p. H and report the color value with the p. H.

Sources of Color • Discharge of highly colored wastewater into water sources. Examples ? Dyeing operation in textile industry. Pulping operation in paper industry lignin. Lignin is highly colored and resistant to biological degredation.

Sources of Color • Natural coloring material Generally not harmful and toxic Yellowish brown color

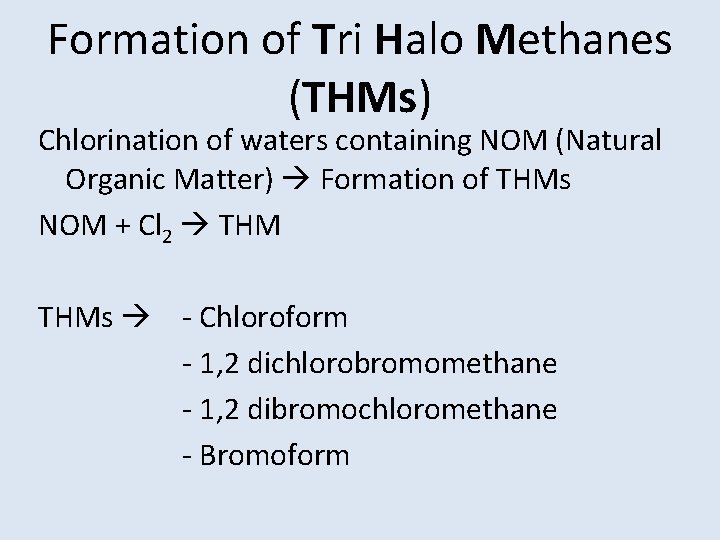
Formation of Tri Halo Methanes (THMs) Chlorination of waters containing NOM (Natural Organic Matter) Formation of THMs NOM + Cl 2 THMs - Chloroform - 1, 2 dichlorobromomethane - 1, 2 dibromochloromethane - Bromoform

Color Standard in Drinking Water • Primary ? Secondary? Aesthetic reasons USEPA drinking water std. Color < 15 units recommended Secondary Maximum Contaminant Level SMCL

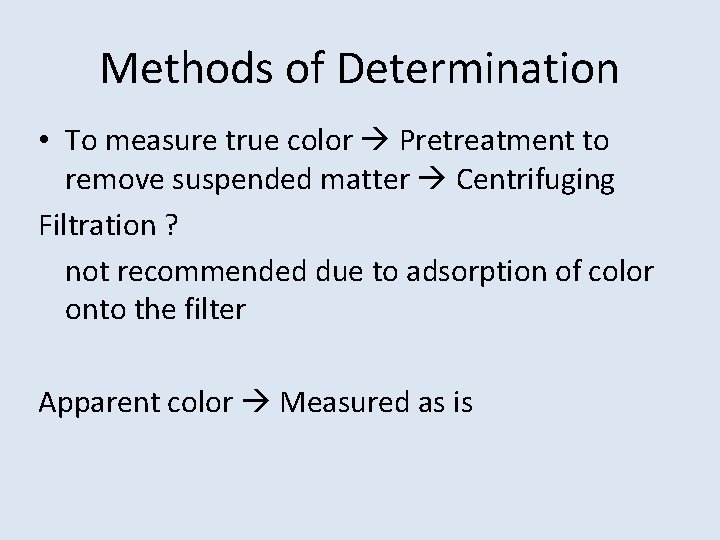
Methods of Determination • To measure true color Pretreatment to remove suspended matter Centrifuging Filtration ? not recommended due to adsorption of color onto the filter Apparent color Measured as is

Standard Color Solutions • Potassium chloro platinate K 2 Pt. Cl 6 tinted with cobalt chloride Yellow – brownish color 1 mg/L platinum K 2 Pt. Cl 6 Standard unit of color

Color Measurement • Color comparison tubes (Nessler tubes) 0 – 70 color units If color > 70 Dilution is necessary • Colored glass disks • Spectrophotometric determination


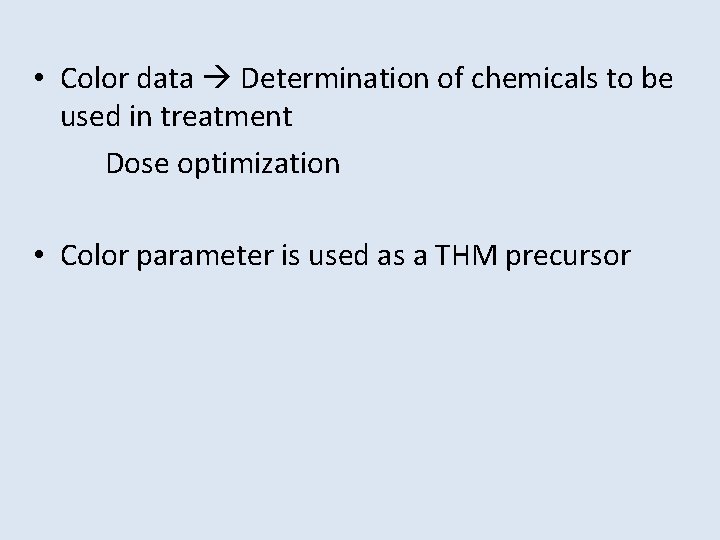
• Color data Determination of chemicals to be used in treatment Dose optimization • Color parameter is used as a THM precursor