Entropy State Function Property with a specific value











- Slides: 18

Entropy

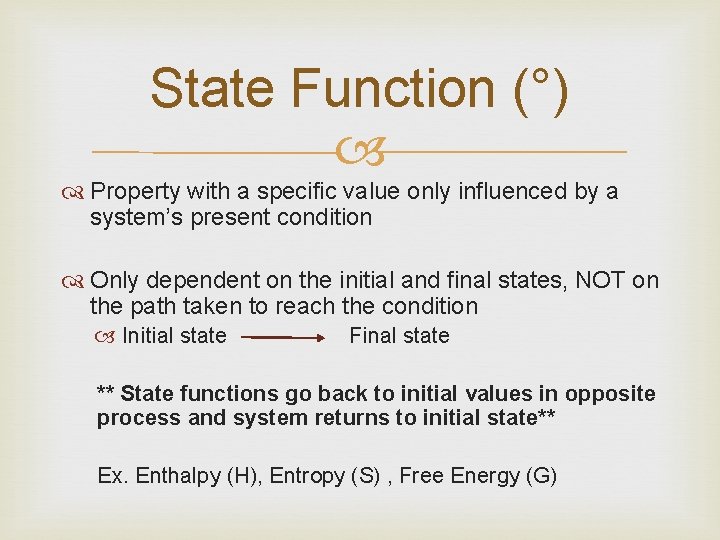
State Function (°) Property with a specific value only influenced by a system’s present condition Only dependent on the initial and final states, NOT on the path taken to reach the condition Initial state Final state ** State functions go back to initial values in opposite process and system returns to initial state** Ex. Enthalpy (H), Entropy (S) , Free Energy (G)

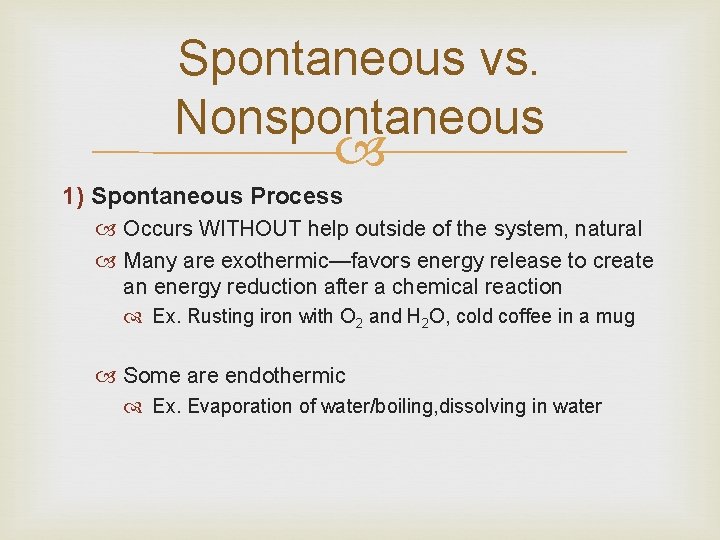
Spontaneous vs. Nonspontaneous 1) Spontaneous Process Occurs WITHOUT help outside of the system, natural Many are exothermic—favors energy release to create an energy reduction after a chemical reaction Ex. Rusting iron with O 2 and H 2 O, cold coffee in a mug Some are endothermic Ex. Evaporation of water/boiling, dissolving in water

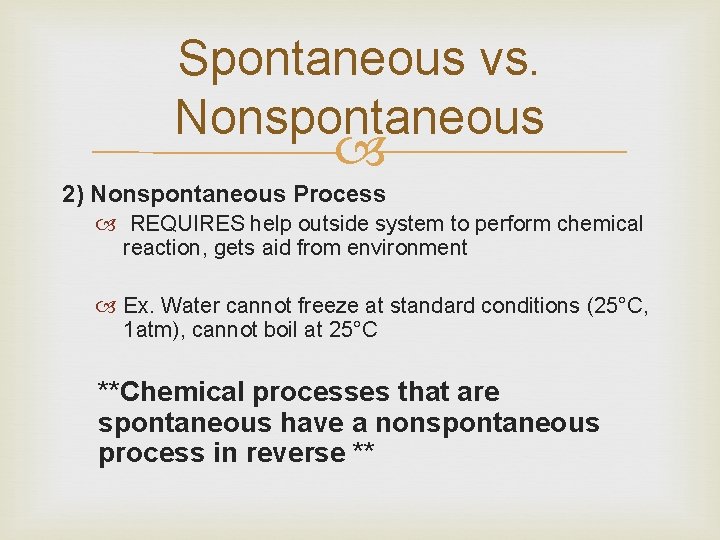
Spontaneous vs. Nonspontaneous 2) Nonspontaneous Process REQUIRES help outside system to perform chemical reaction, gets aid from environment Ex. Water cannot freeze at standard conditions (25°C, 1 atm), cannot boil at 25°C **Chemical processes that are spontaneous have a nonspontaneous process in reverse **

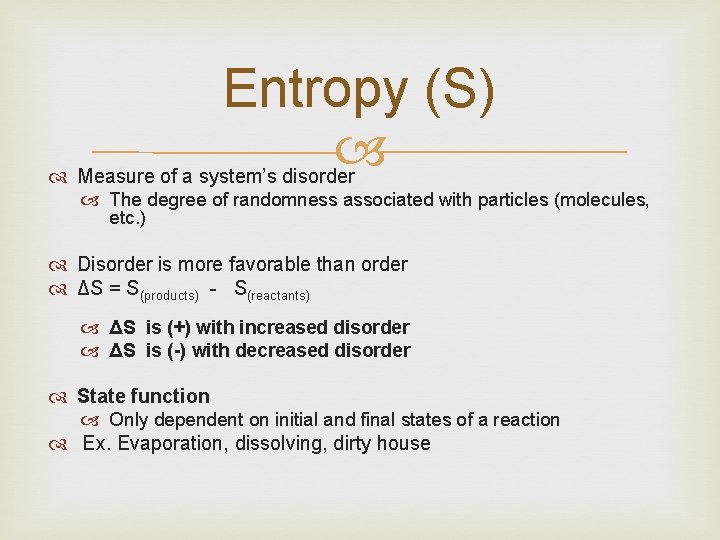
Entropy (S) Measure of a system’s disorder The degree of randomness associated with particles (molecules, etc. ) Disorder is more favorable than order ΔS = S(products) - S(reactants) ΔS is (+) with increased disorder ΔS is (-) with decreased disorder State function Only dependent on initial and final states of a reaction Ex. Evaporation, dissolving, dirty house

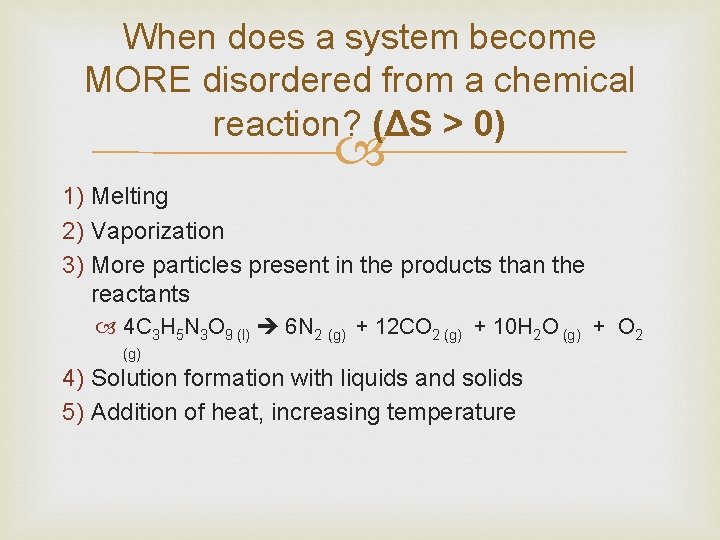
When does a system become MORE disordered from a chemical reaction? (ΔS > 0) 1) Melting 2) Vaporization 3) More particles present in the products than the reactants 4 C 3 H 5 N 3 O 9 (l) 6 N 2 (g) + 12 CO 2 (g) + 10 H 2 O (g) + O 2 (g) 4) Solution formation with liquids and solids 5) Addition of heat, increasing temperature

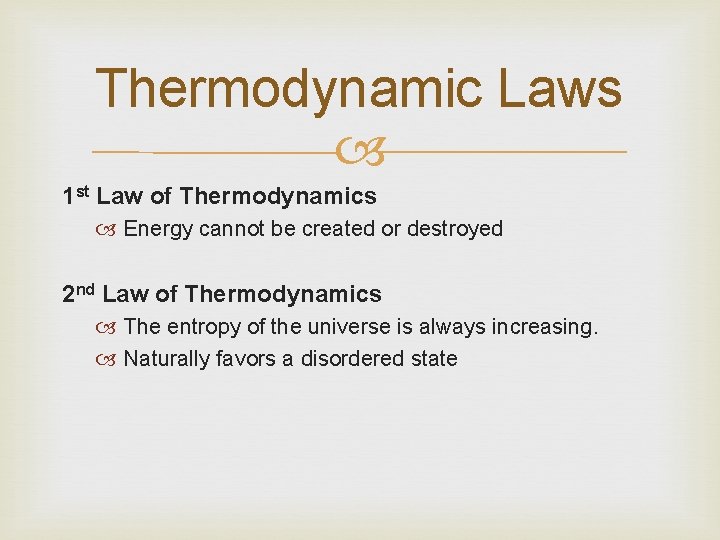
Thermodynamic Laws 1 st Law of Thermodynamics Energy cannot be created or destroyed 2 nd Law of Thermodynamics The entropy of the universe is always increasing. Naturally favors a disordered state

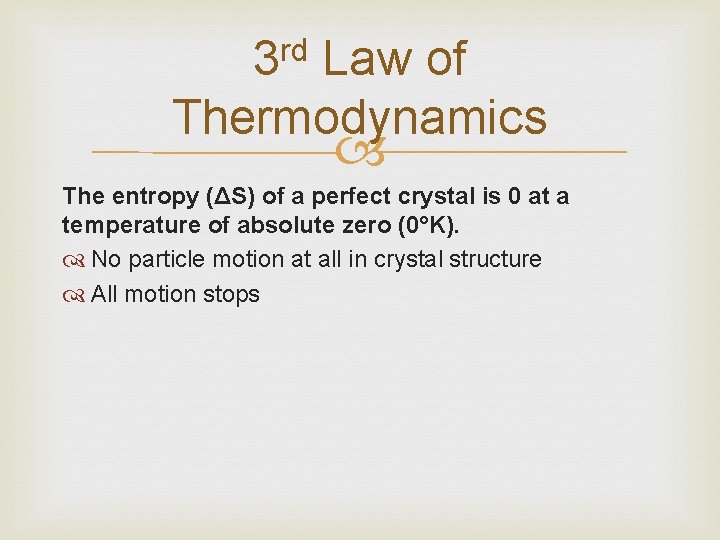
3 rd Law of Thermodynamics The entropy (ΔS) of a perfect crystal is 0 at a temperature of absolute zero (0°K). No particle motion at all in crystal structure All motion stops

How do we determine if a chemical reaction is spontaneous? 1) Change in entropy (ΔS) 2) Gibbs Free Energy (ΔG)

Gibbs Free Energy

Gibbs Free Energy (G) Balances the relationship between enthalpy (ΔH) and entropy (ΔS) State function Enthalpy of system minus the product of temperature times entropy of system G = H – TS Maximum amount of energy available to do work, “free”

Change in Gibbs Free Energy (ΔG) ΔG = ΔH – TΔS Relates enthalpy and entropy to determine which has more importance in determining whether a reaction is spontaneous Combines energy transfer as heat (ΔH) and energy released to contribute to disorder (ΔS)

Gibbs Free Energy DG is the change in Gibbs free energy. DG can be calculated as DGo = DHo - TDSo The term DH represents enthalpy or heat energy which is available to do work. The term DS represents entropy or random motion which is not available to do work.

Example 2: Find ΔG for a chemical reaction given ΔH = -218 k. J and ΔS = -765 J/K at 32°C.

Change in Gibbs Free Energy (ΔG) ΔG = ΔH – TΔS ΔG < 0 , spontaneous reaction, reaction occurs as written Energy available to do work ΔG > 0, nonspontaneous reaction, reaction will NOT occur as written Energy deficiency, no leftover energy and not enough energy for reaction ** All reactions want to move toward low or minimal ΔG

A spontaneous reaction is NOT necessarily fast!!!! Reaction rate involves kinetics ! !

What makes a reaction spontaneous? Entropy(ΔS) > 0, POSITIVE Reaction creates more disorder Free Energy (ΔG) < 0, NEGATIVE

Homework Read pp. 546 -549 p. 550 #2 -4, 5