Entropy S Entropy is disorder randomness dispersal of
![Entropy (J/K) [heat entering system at given T] convert q to S System 1 Entropy (J/K) [heat entering system at given T] convert q to S System 1](https://slidetodoc.com/presentation_image_h2/9eea0ae15fcd8065757c3386aadb0dd5/image-7.jpg)
- Slides: 13

Entropy = S Entropy is disorder randomness dispersal of energy

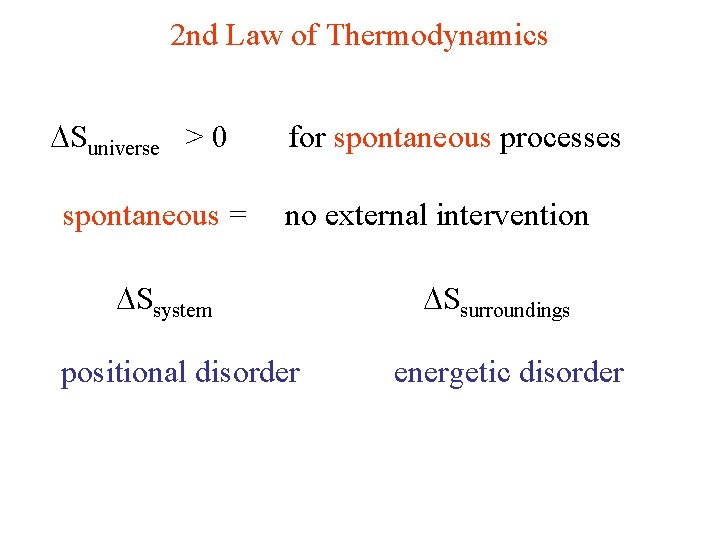
2 nd Law of Thermodynamics Suniverse > 0 spontaneous = for spontaneous processes no external intervention Ssystem positional disorder Ssurroundings energetic disorder

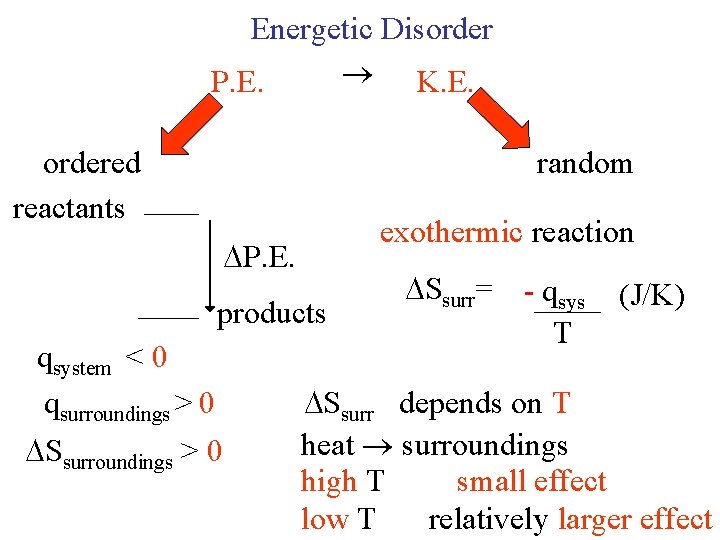
Energetic Disorder P. E. a) K. E. b) ordered reactants random a) endothermic reaction b) exothermic reaction P. E. products qsystem < 0 qsurroundings > 0 Ssurroundings > 0 Ssurr= - qsys T (J/K) Ssurr depends on T heat surroundings high T small effect low T relatively larger effect

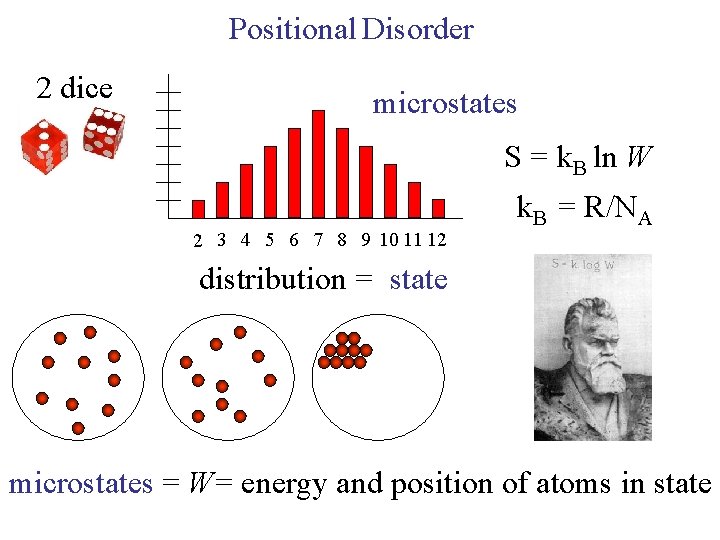
Positional Disorder 2 dice microstates S = k. B ln W 2 3 4 5 6 7 8 9 10 11 12 k. B = R/NA distribution = state microstates = W= energy and position of atoms in state

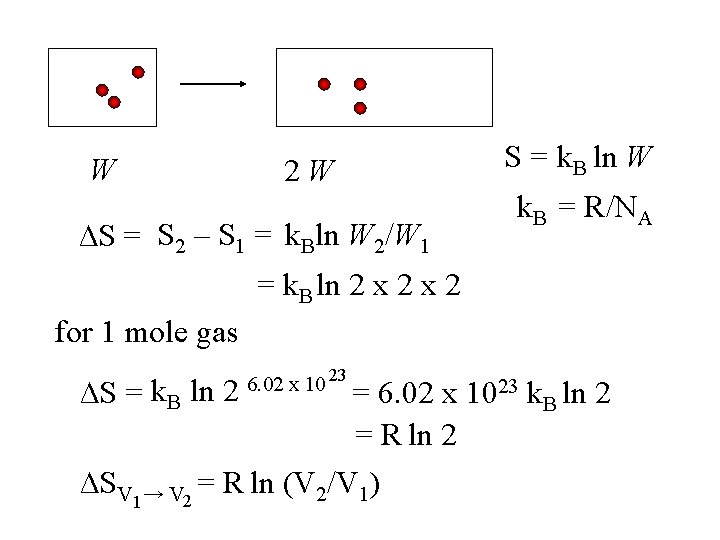
W 2 W ∆S = S 2 – S 1 = k. Bln W 2/W 1 S = k. B ln W k. B = R/NA = k. B ln 2 x 2 for 1 mole gas ∆S = k. B ln 2 6. 02 x 10 23 = 6. 02 x 1023 k. B ln 2 = R ln 2 ∆SV 1→ V 2 = R ln (V 2/V 1)

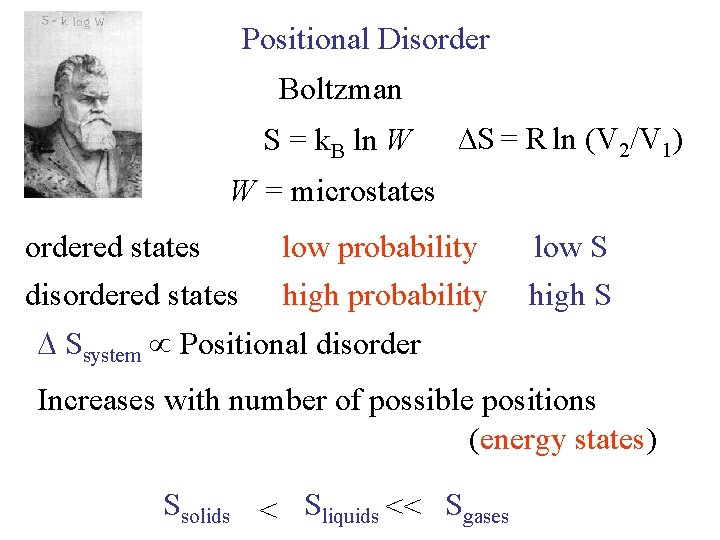
Positional Disorder Boltzman S = k. B ln W ∆S = R ln (V 2/V 1) W = microstates ordered states disordered states low probability high probability low S high S Ssystem Positional disorder Increases with number of possible positions (energy states) Ssolids < Sliquids << Sgases
![Entropy JK heat entering system at given T convert q to S System 1 Entropy (J/K) [heat entering system at given T] convert q to S System 1](https://slidetodoc.com/presentation_image_h2/9eea0ae15fcd8065757c3386aadb0dd5/image-7.jpg)
Entropy (J/K) [heat entering system at given T] convert q to S System 1 Pext = 1. 5 atm w = -182 J q = +182 J T = 298 K System 2 Pext = 0 atm w=0 q=0 E = 0

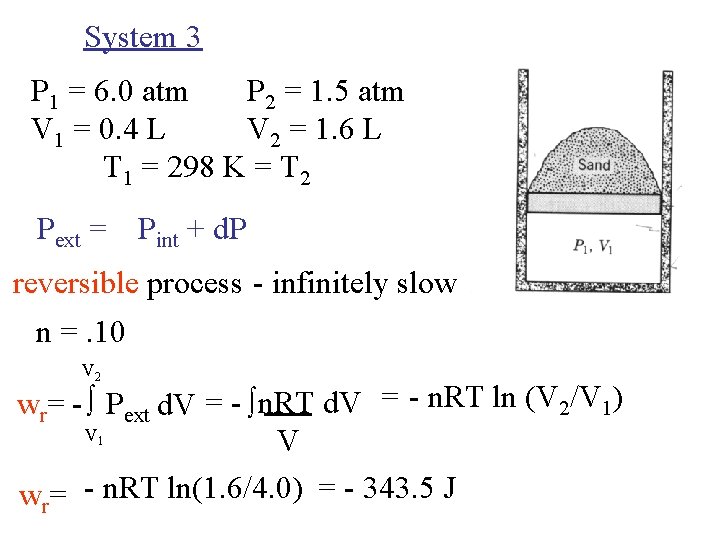
System 3 P 1 = 6. 0 atm P 2 = 1. 5 atm V 1 = 0. 4 L V 2 = 1. 6 L T 1 = 298 K = T 2 Pext = Pint + d. P reversible process - infinitely slow n =. 10 V 2 wr= - Pext d. V = - n. RT d. V = - n. RT ln (V 2/V 1) V V wr= - n. RT ln(1. 6/4. 0) = - 343. 5 J 1

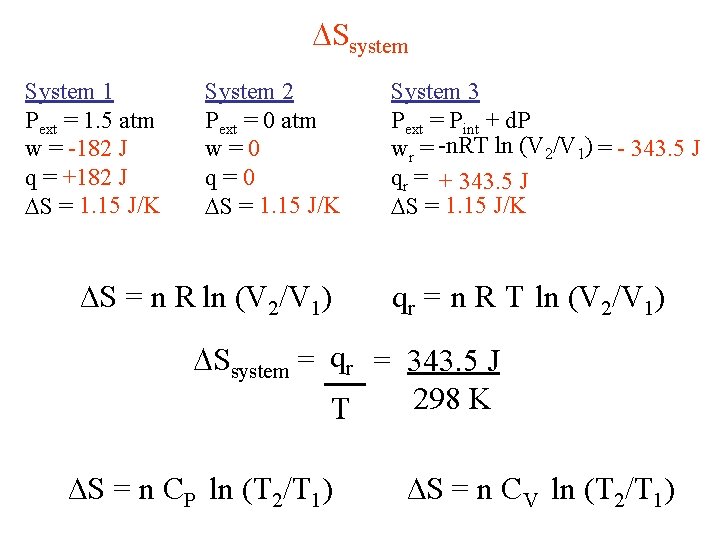
Ssystem System 1 Pext = 1. 5 atm w = -182 J q = +182 J S = 1. 15 J/K System 2 Pext = 0 atm w=0 q=0 S = 1. 15 J/K ∆S = n R ln (V 2/V 1) System 3 Pext = Pint + d. P wr = -n. RT ln (V 2/V 1) = - 343. 5 J qr = + 343. 5 J S = 1. 15 J/K qr = n R T ln (V 2/V 1) Ssystem = qr = 343. 5 J 298 K T ∆S = n CP ln (T 2/T 1) ∆S = n CV ln (T 2/T 1)

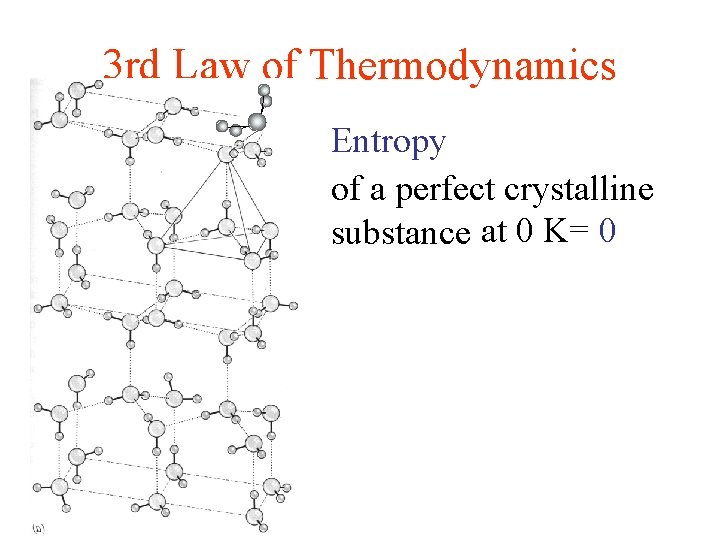
3 rd Law of Thermodynamics Entropy of a perfect crystalline substance at 0 K= 0

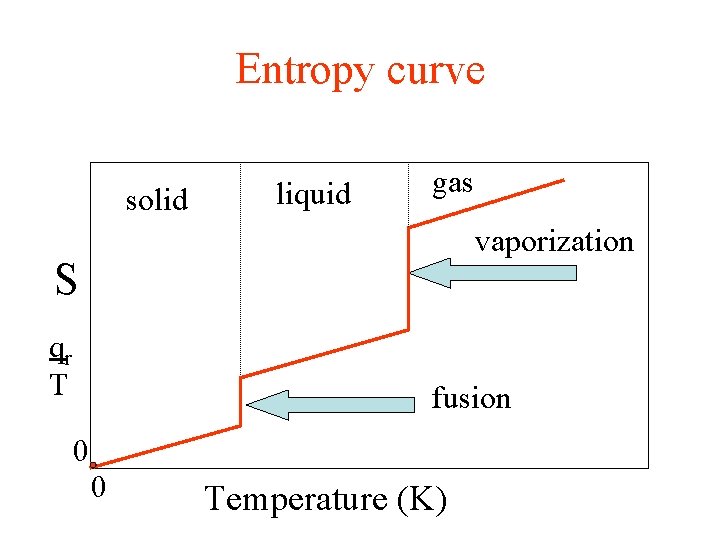
Entropy curve solid liquid gas vaporization S qr T fusion 0 0 Temperature (K)

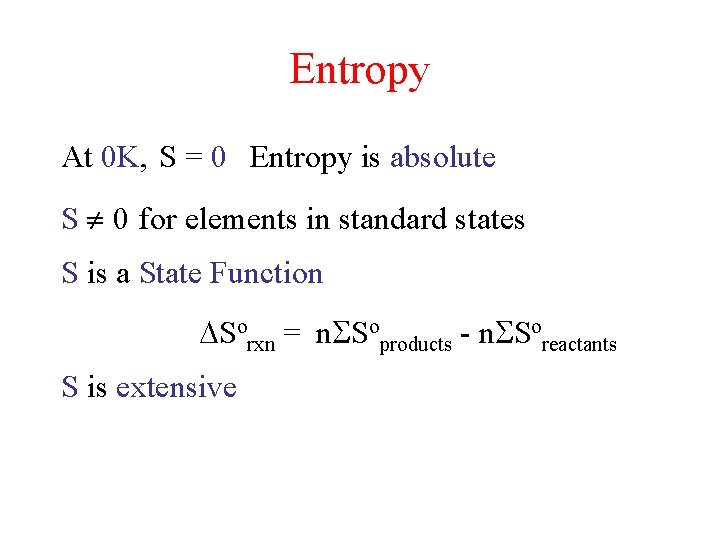
Entropy At 0 K, S = 0 Entropy is absolute S 0 for elements in standard states S is a State Function Sorxn = n Soproducts - n Soreactants S is extensive

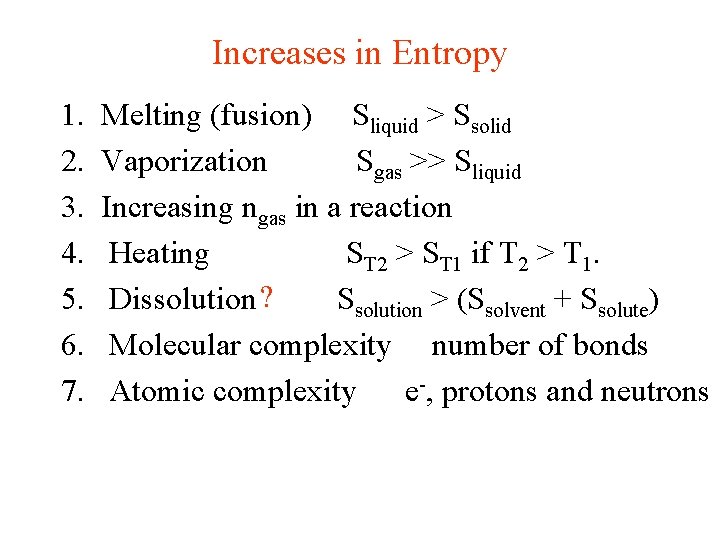
Increases in Entropy 1. 2. 3. 4. 5. 6. 7. Melting (fusion) Sliquid > Ssolid Vaporization Sgas >> Sliquid Increasing ngas in a reaction Heating ST 2 > ST 1 if T 2 > T 1. Dissolution ? Ssolution > (Ssolvent + Ssolute) Molecular complexity number of bonds Atomic complexity e-, protons and neutrons