Entropy and the Second Law of Thermodynamics Heat








- Slides: 32

Entropy and the Second Law of Thermodynamics

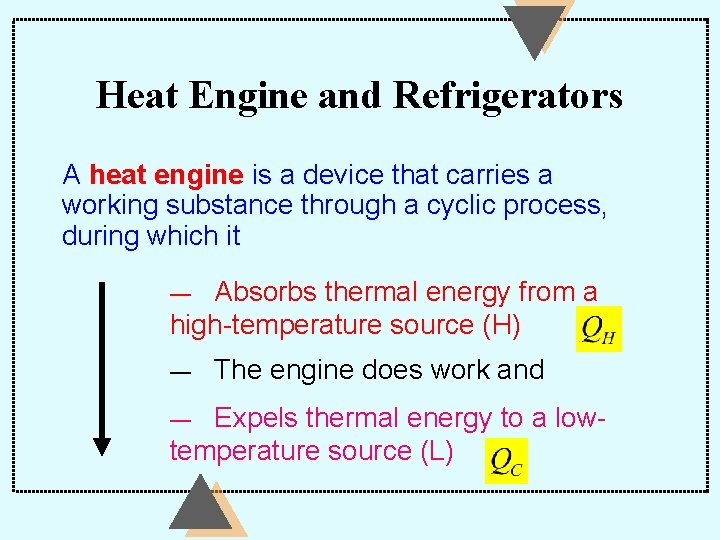
Heat Engine and Refrigerators A heat engine is a device that carries a working substance through a cyclic process, during which it Absorbs thermal energy from a high-temperature source (H) — — The engine does work and Expels thermal energy to a lowtemperature source (L) —

1 st Law Cyclic Process Work done by the substance Thermal efficiency: Heat added to the substance

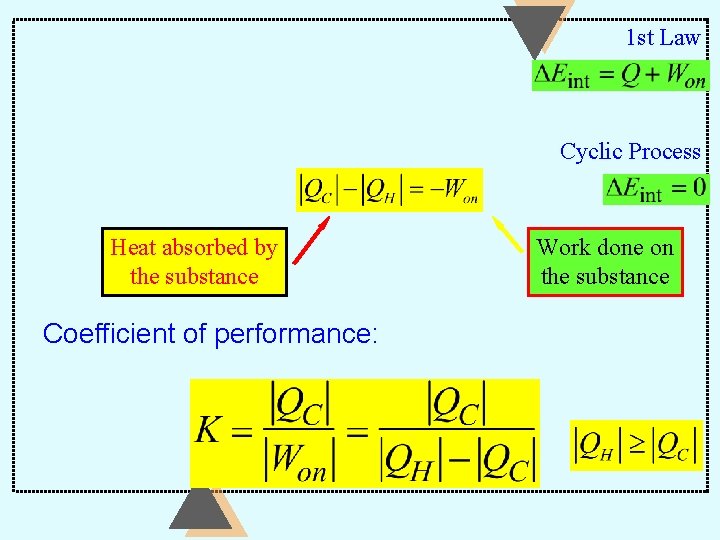
A refrigerator is a heat engine in reverse. A refrigerator is a device that carries a working substance through a cyclic process, during which it Absorbs thermal energy from a low-temperature source — — by doing Work Expels thermal energy (the amount work done and the heat absorbed) to a high-temperature source —

1 st Law Cyclic Process Heat absorbed by the substance Coefficient of performance: Work done on the substance

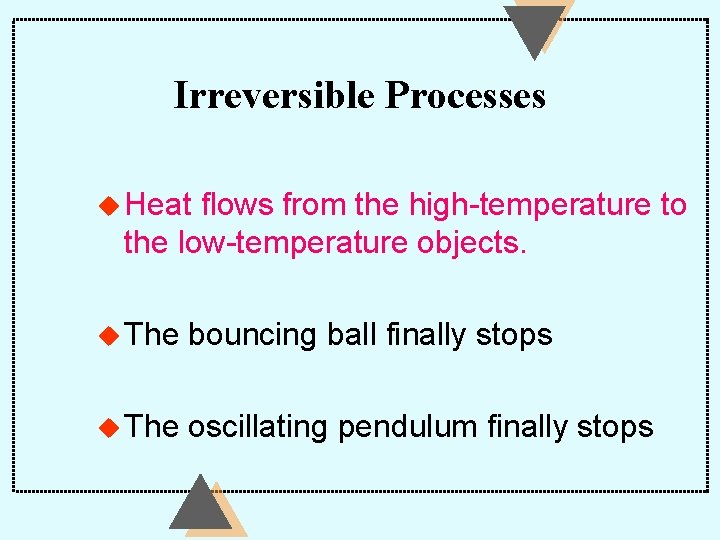
Reversible and Irreversible Processes Reversible Process: a process that at the conclusion, the system and its surrounding return to the exact initial conditions. All natural processes are irreversible, at best “almost” reversible.

Irreversible Processes u Heat flows from the high-temperature to the low-temperature objects. u The bouncing ball finally stops u The oscillating pendulum finally stops

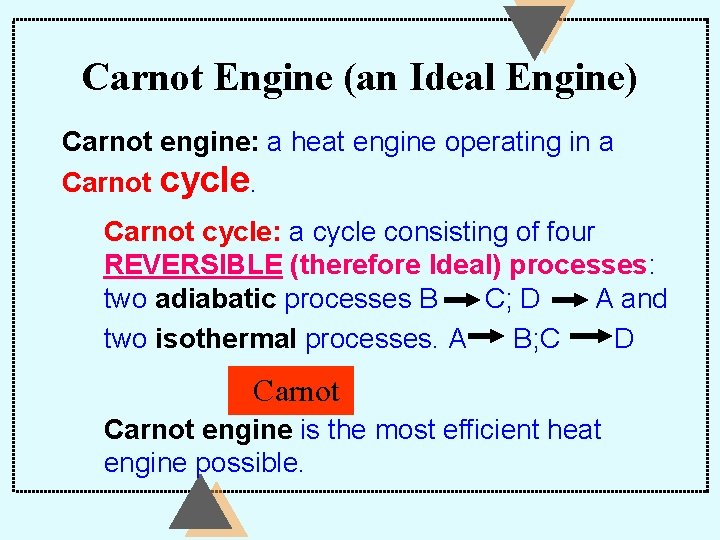
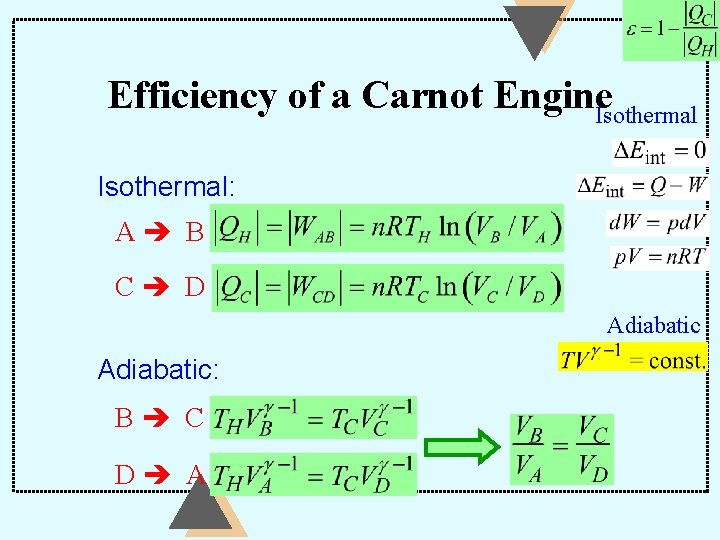
Carnot Engine (an Ideal Engine) Carnot engine: a heat engine operating in a Carnot cycle: a cycle consisting of four REVERSIBLE (therefore Ideal) processes: two adiabatic processes B C; D A and two isothermal processes. A B; C D Carnot engine is the most efficient heat engine possible.

Efficiency of a Carnot Engine. Isothermal: A B C D Adiabatic: B C D A

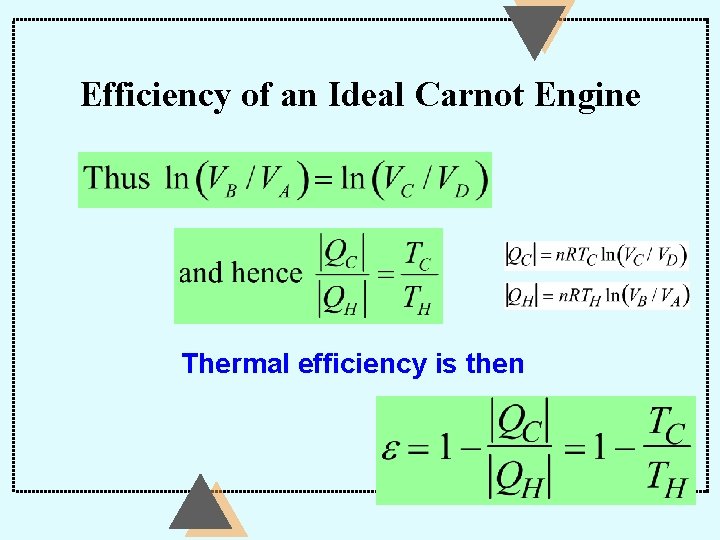
Efficiency of an Ideal Carnot Engine Thermal efficiency is then

Performance of an Ideal Carnot Refrigerator Coefficient of performance

HRW 38 E (5 th ed. ). An ideal heat engine operates in a Carnot cycle between 235˚C and 115˚C. It absorbs 6. 30 x 104 J per cycle at the higher temperature. (a) What is the efficiency of the engine? (b) How much work per cycle is this engine capable of performing? (a) TH - TC (235 - 115) K e= = = 23. 6% TH (235 + 273) K

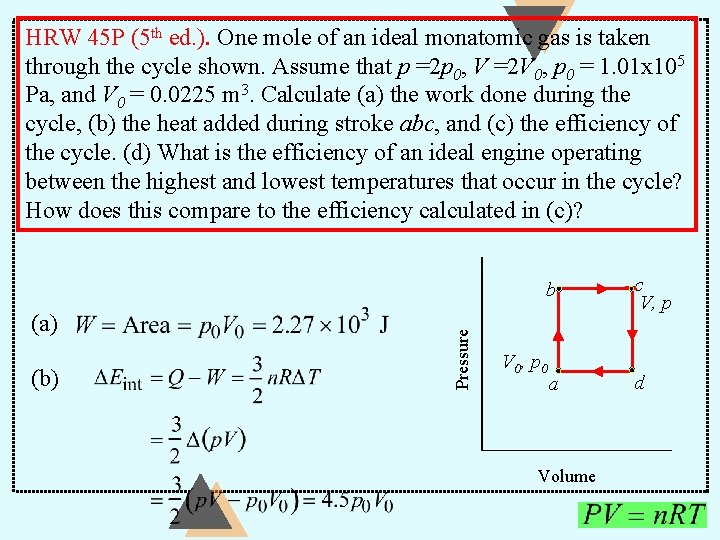
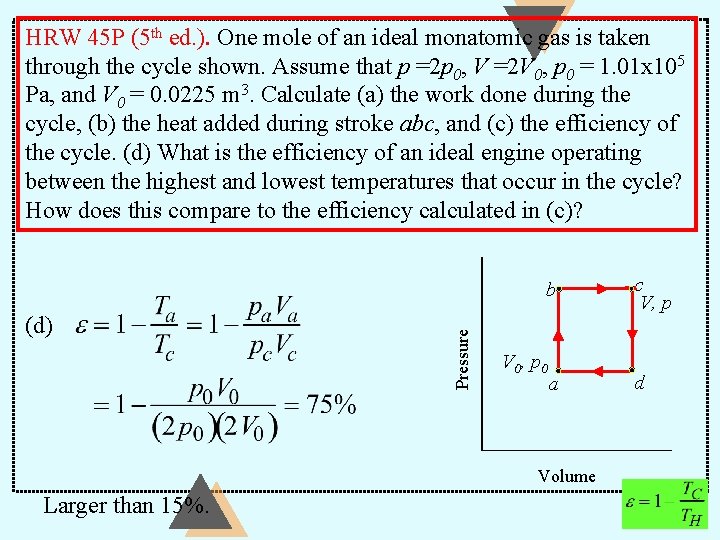
HRW 45 P (5 th ed. ). One mole of an ideal monatomic gas is taken through the cycle shown. Assume that p =2 p 0, V =2 V 0, p 0 = 1. 01 x 105 Pa, and V 0 = 0. 0225 m 3. Calculate (a) the work done during the cycle, (b) the heat added during stroke abc, and (c) the efficiency of the cycle. (d) What is the efficiency of an ideal engine operating between the highest and lowest temperatures that occur in the cycle? How does this compare to the efficiency calculated in (c)? (a) (b) Pressure b V 0, p 0 a Volume c V, p d

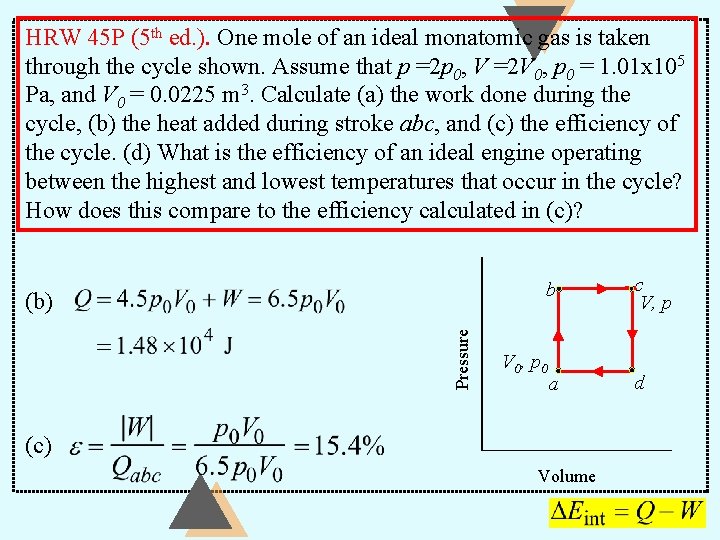
HRW 45 P (5 th ed. ). One mole of an ideal monatomic gas is taken through the cycle shown. Assume that p =2 p 0, V =2 V 0, p 0 = 1. 01 x 105 Pa, and V 0 = 0. 0225 m 3. Calculate (a) the work done during the cycle, (b) the heat added during stroke abc, and (c) the efficiency of the cycle. (d) What is the efficiency of an ideal engine operating between the highest and lowest temperatures that occur in the cycle? How does this compare to the efficiency calculated in (c)? b Pressure (b) V 0, p 0 a (c) Volume c V, p d

HRW 45 P (5 th ed. ). One mole of an ideal monatomic gas is taken through the cycle shown. Assume that p =2 p 0, V =2 V 0, p 0 = 1. 01 x 105 Pa, and V 0 = 0. 0225 m 3. Calculate (a) the work done during the cycle, (b) the heat added during stroke abc, and (c) the efficiency of the cycle. (d) What is the efficiency of an ideal engine operating between the highest and lowest temperatures that occur in the cycle? How does this compare to the efficiency calculated in (c)? (d) Pressure b V 0, p 0 a Volume Larger than 15%. c V, p d

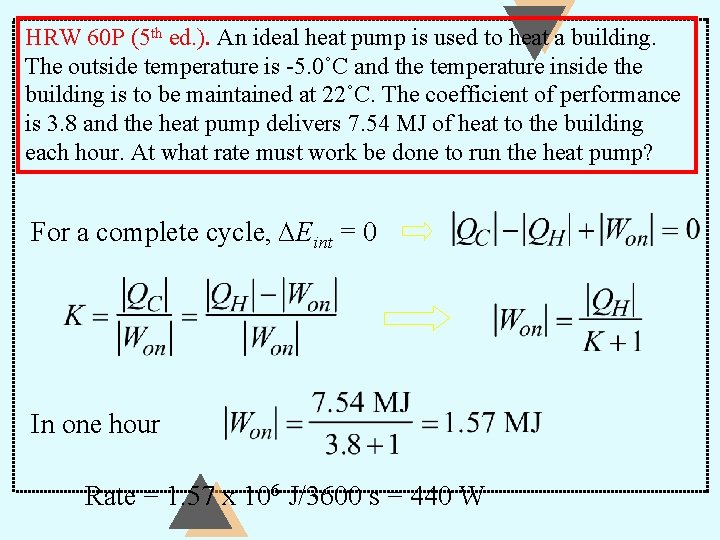
HRW 60 P (5 th ed. ). An ideal heat pump is used to heat a building. The outside temperature is -5. 0˚C and the temperature inside the building is to be maintained at 22˚C. The coefficient of performance is 3. 8 and the heat pump delivers 7. 54 MJ of heat to the building each hour. At what rate must work be done to run the heat pump? For a complete cycle, ∆Eint = 0 In one hour Rate = 1. 57 x 106 J/3600 s = 440 W

Entropy and the Second Law of Thermodynamics

The Second Law of Thermodynamics It is impossible to construct a heat engine with 100% efficiency. It is impossible to construct a refrigerator that does not require work.

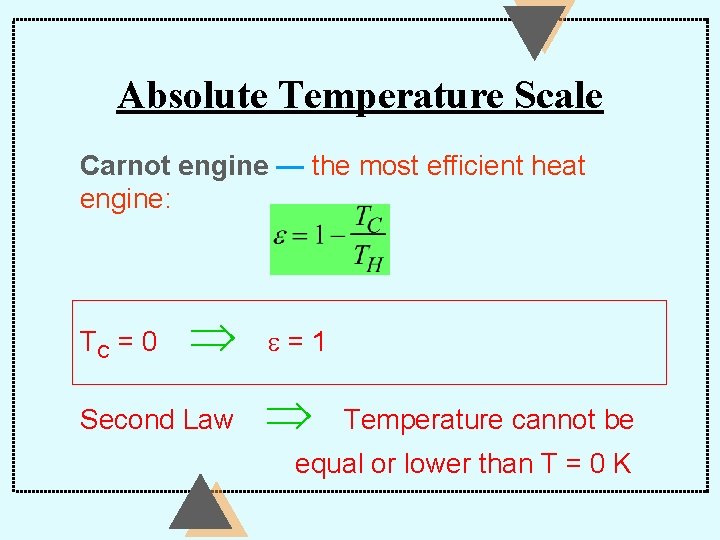
Absolute Temperature Scale Carnot engine — the most efficient heat engine: TC = 0 Second Law e=1 Temperature cannot be equal or lower than T = 0 K

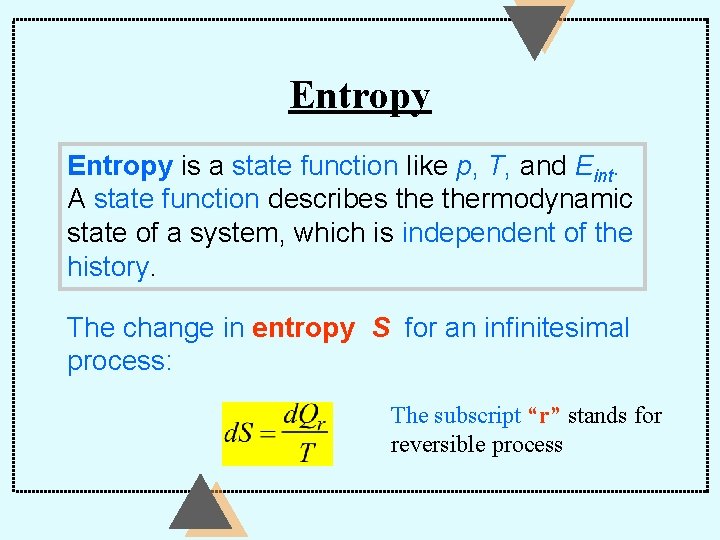
Entropy is a state function like p, T, and Eint. A state function describes thermodynamic state of a system, which is independent of the history. The change in entropy S for an infinitesimal process: The subscript “r” stands for reversible process

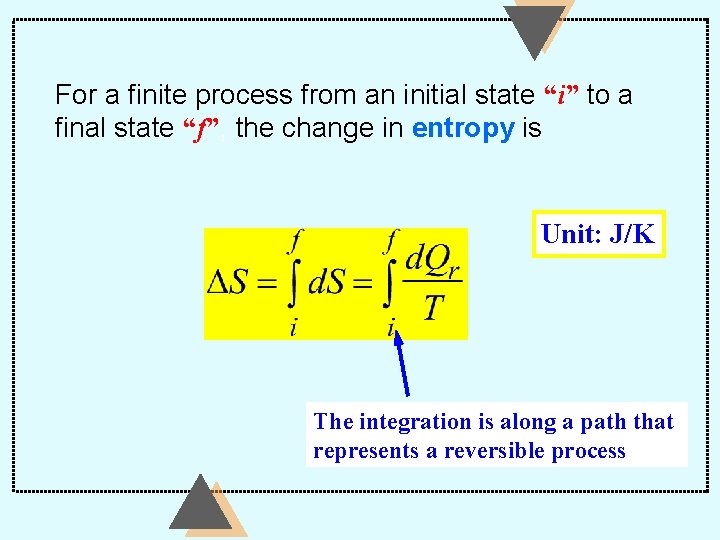
For a finite process from an initial state “i” to a final state “f”, the change in entropy is Unit: J/K The integration is along a path that represents a reversible process

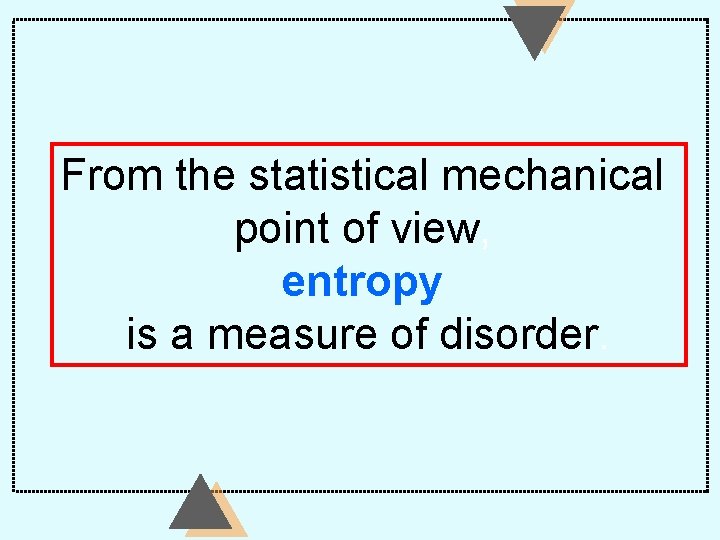
From the statistical mechanical point of view, entropy is a measure of disorder.

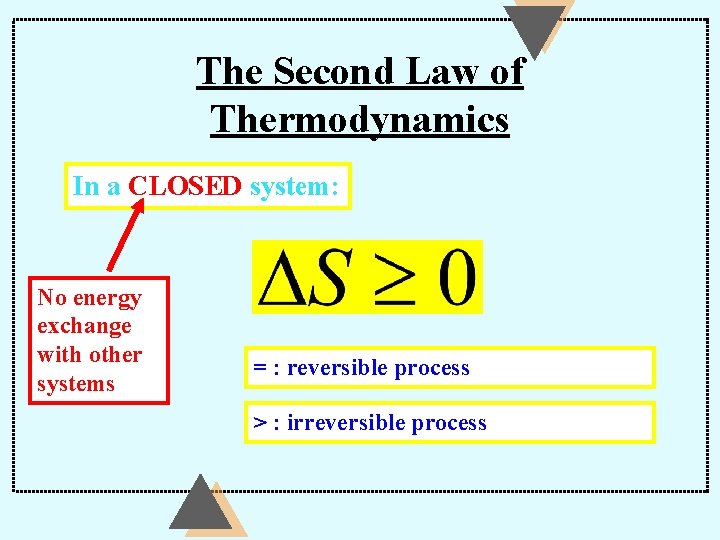
The Second Law of Thermodynamics In a CLOSED system: No energy exchange with other systems = : reversible process > : irreversible process

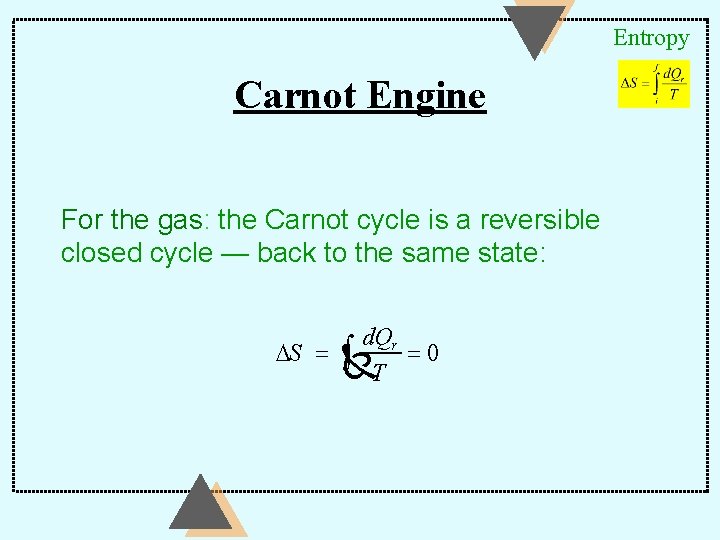
Entropy Carnot Engine For the gas: the Carnot cycle is a reversible closed cycle — back to the same state: d. Qr DS = Ñ ò T =0

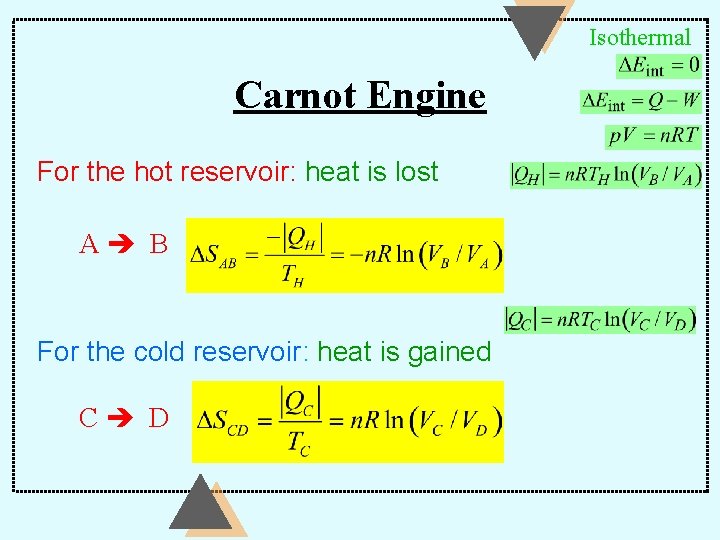
Isothermal Carnot Engine For the hot reservoir: heat is lost A B For the cold reservoir: heat is gained C D

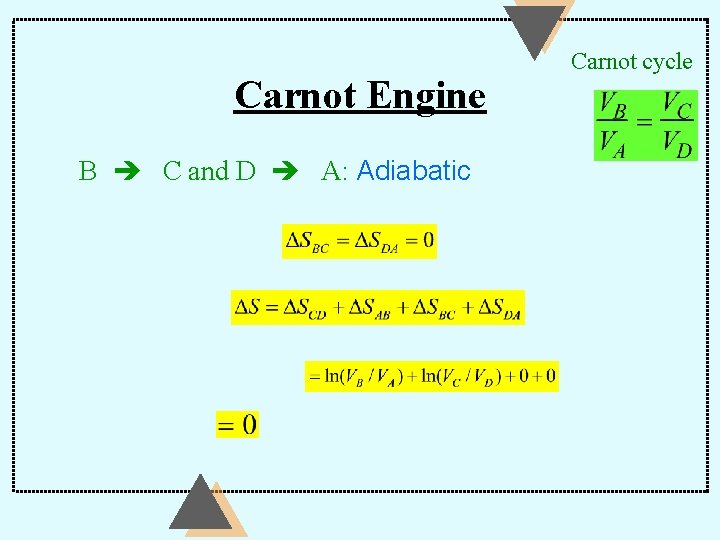
Carnot Engine B C and D A: Adiabatic Carnot cycle

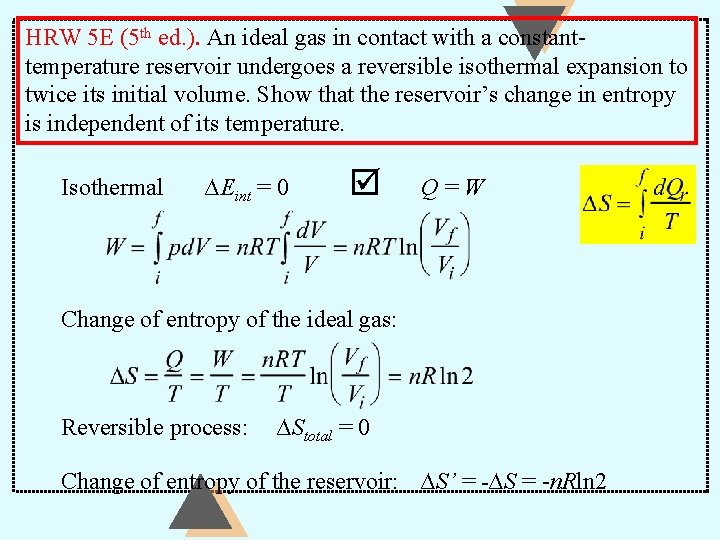
HRW 5 E (5 th ed. ). An ideal gas in contact with a constanttemperature reservoir undergoes a reversible isothermal expansion to twice its initial volume. Show that the reservoir’s change in entropy is independent of its temperature. Isothermal ∆Eint = 0 Q=W Change of entropy of the ideal gas: Reversible process: ∆Stotal = 0 Change of entropy of the reservoir: ∆S’ = -∆S = -n. Rln 2

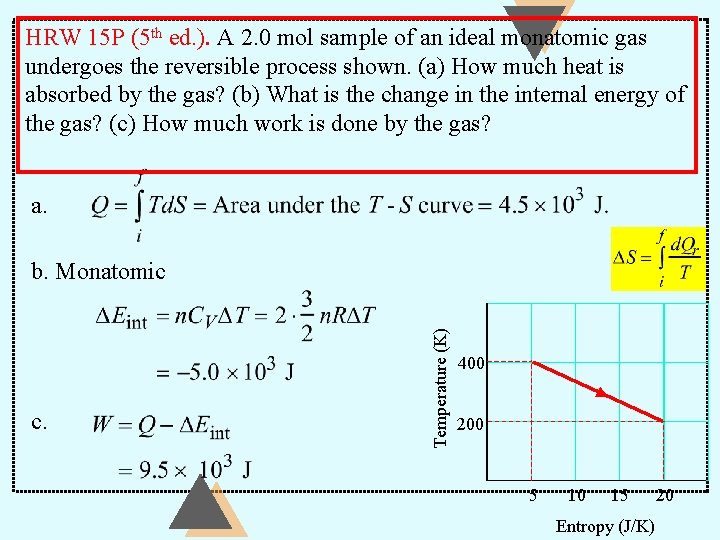
HRW 15 P (5 th ed. ). A 2. 0 mol sample of an ideal monatomic gas undergoes the reversible process shown. (a) How much heat is absorbed by the gas? (b) What is the change in the internal energy of the gas? (c) How much work is done by the gas? a. c. Temperature (K) b. Monatomic 400 200 5 10 15 Entropy (J/K) 20

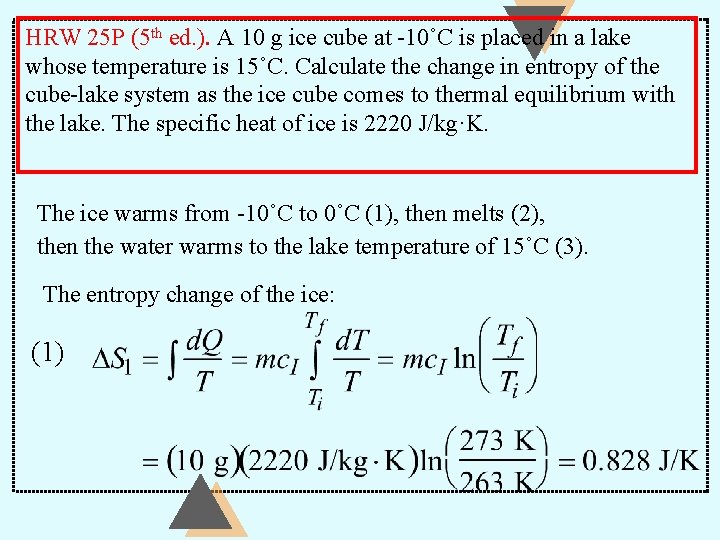
HRW 25 P (5 th ed. ). A 10 g ice cube at -10˚C is placed in a lake whose temperature is 15˚C. Calculate the change in entropy of the cube-lake system as the ice cube comes to thermal equilibrium with the lake. The specific heat of ice is 2220 J/kg·K. The ice warms from -10˚C to 0˚C (1), then melts (2), then the water warms to the lake temperature of 15˚C (3). The entropy change of the ice: (1)

HRW 25 P (5 th ed. ). A 10 g ice cube at -10˚C is placed in a lake whose temperature is 15˚C. Calculate the change in entropy of the cube-lake system as the ice cube comes to thermal equilibrium with the lake. The specific heat of ice is 2220 J/kg·K. (2) (3)

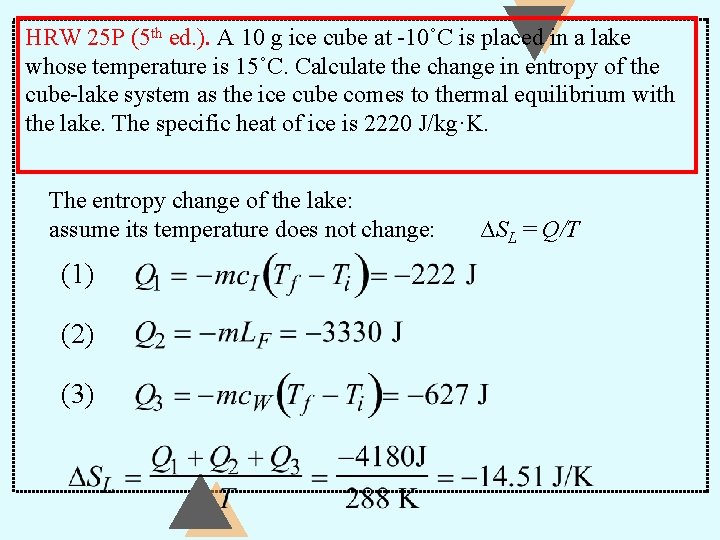
HRW 25 P (5 th ed. ). A 10 g ice cube at -10˚C is placed in a lake whose temperature is 15˚C. Calculate the change in entropy of the cube-lake system as the ice cube comes to thermal equilibrium with the lake. The specific heat of ice is 2220 J/kg·K. The entropy change of the lake: assume its temperature does not change: (1) (2) (3) ∆SL = Q/T

HRW 25 P (5 th ed. ). A 10 g ice cube at -10˚C is placed in a lake whose temperature is 15˚C. Calculate the change in entropy of the cube-lake system as the ice cube comes to thermal equilibrium with the lake. The specific heat of ice is 2220 J/kg·K.
 Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law
Newton's first law Kelvin statement
Kelvin statement State second law of thermodynamics
State second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics 2nd law of thermodynamics
2nd law of thermodynamics 2 nd law of thermodynamics
2 nd law of thermodynamics State the second law of thermodynamics
State the second law of thermodynamics Thermodynamics
Thermodynamics Total entropy
Total entropy Thermodynamic second law
Thermodynamic second law Entropy and heat transfer
Entropy and heat transfer Entropy of ideal gas
Entropy of ideal gas Law of entropy
Law of entropy Thermodynamics enthalpy of reaction and hess's law
Thermodynamics enthalpy of reaction and hess's law 27 miles per gallon into kilometers per liter
27 miles per gallon into kilometers per liter Energy balance thermodynamics
Energy balance thermodynamics Zeroth law of thermo
Zeroth law of thermo Third law of thermodynamics derivation
Third law of thermodynamics derivation Thermodynamics laws
Thermodynamics laws Isobaric process formula
Isobaric process formula Brayton cycle process
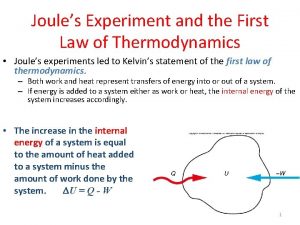
Brayton cycle process First law of thermodynamics experiment
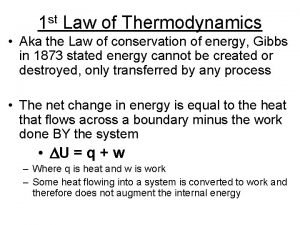
First law of thermodynamics experiment 1st law of thermodynamics
1st law of thermodynamics Steady flow process in thermodynamics
Steady flow process in thermodynamics Laws of thermodynamics
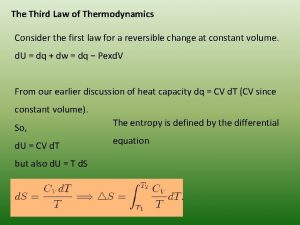
Laws of thermodynamics Third law of thermodynamics is depend on
Third law of thermodynamics is depend on Dq=cdt
Dq=cdt First law of thermodynamics sign convention
First law of thermodynamics sign convention 1st law of thermodynamics
1st law of thermodynamics 1st law of thermodynamics
1st law of thermodynamics First law of thermodynamics control mass
First law of thermodynamics control mass