Entropy a measure of the number of ways

Entropy a measure of the number of ways that molecules and their associated energy can be arranged Stepehn Brown 2013
What is entropy? • Energy tends to spread out among the molecules in a system • Molecules are more likely to be in an arrangement where energy is widely distributed – we call this disorder • This is the Second Law of Thermodynamics Entropy always increases Stepehn Brown 2013

If I toss a series of coins, will I get all heads, or half heads, half tails? • If you have two coins, there is one way to get two heads, and two ways to get one • For ten coins, there is still only one way to get ten heads, but 252 ways to get five • For twenty coins, it becomes one and 184, 756 • For one hundred coins, it is one and 101 000 000 000 • Disordered systems (half heads, half tails) are much more likely than ordered ones Stepehn Brown 2013

Entropy Change • It is possible to calculate the absolute entropy of a substance S ᴓ (or S under standard conditions) • Given S values for each substance in a reaction, we can calculate ΔS for a reaction: ΔS = S(products) – S(reactants) Stepehn Brown 2013
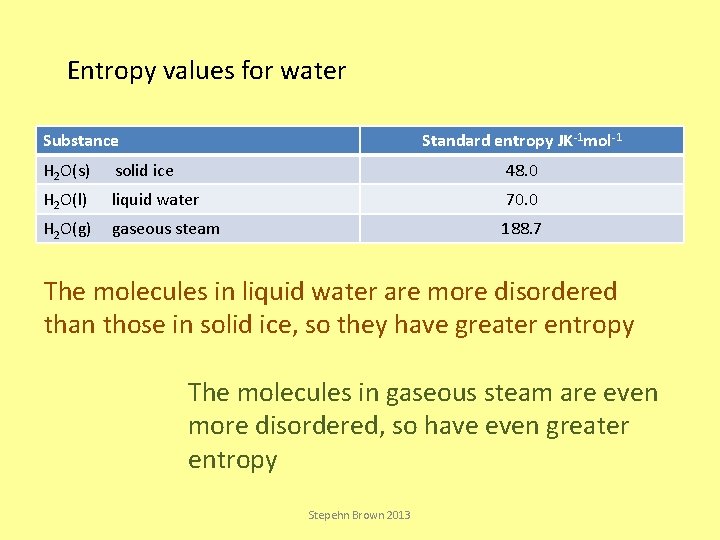
Entropy values for water Substance Standard entropy JK-1 mol-1 H 2 O(s) solid ice 48. 0 H 2 O(l) liquid water 70. 0 H 2 O(g) gaseous steam 188. 7 The molecules in liquid water are more disordered than those in solid ice, so they have greater entropy The molecules in gaseous steam are even more disordered, so have even greater entropy Stepehn Brown 2013
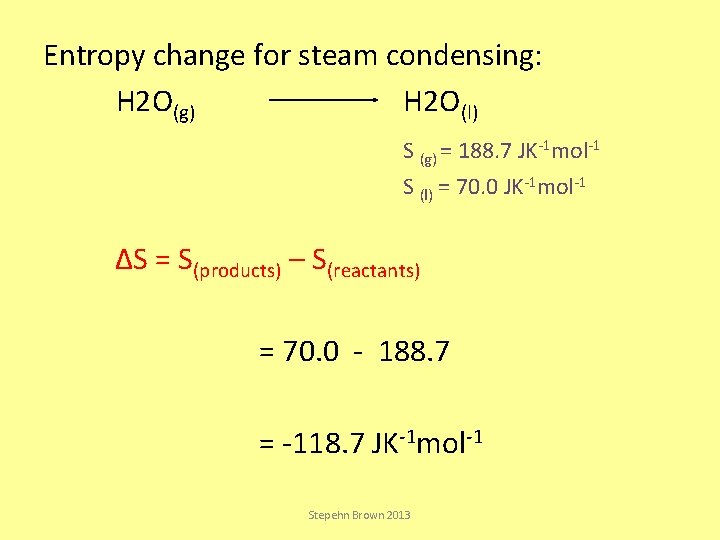
Entropy change for steam condensing: H 2 O(g) H 2 O(l) S (g) = 188. 7 JK-1 mol-1 S (l) = 70. 0 JK-1 mol-1 ΔS = S(products) – S(reactants) = 70. 0 - 188. 7 = -118. 7 JK-1 mol-1 Stepehn Brown 2013

But… • The Second Law of Thermodynamics tells us that ΔS must be positive for any change. • How can steam condensing have a value of ΔS = -118. 7 JK-1 mol-1? Stepehn Brown 2013
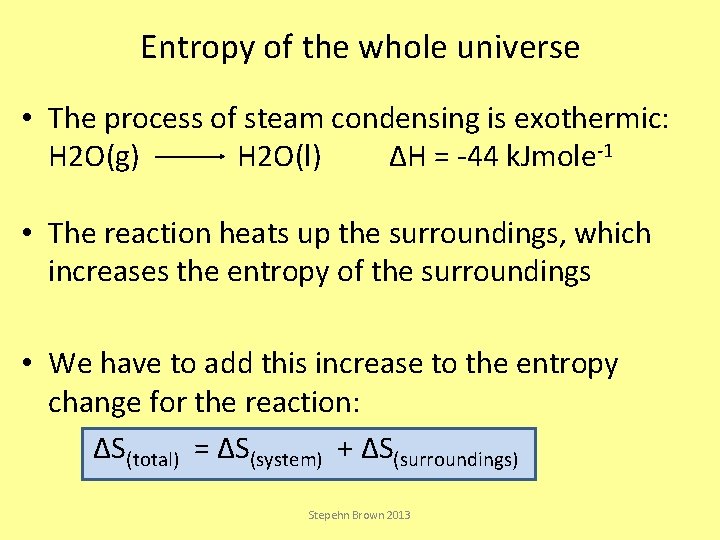
Entropy of the whole universe • The process of steam condensing is exothermic: H 2 O(g) H 2 O(l) ΔH = -44 k. Jmole-1 • The reaction heats up the surroundings, which increases the entropy of the surroundings • We have to add this increase to the entropy change for the reaction: ΔS(total) = ΔS(system) + ΔS(surroundings) Stepehn Brown 2013
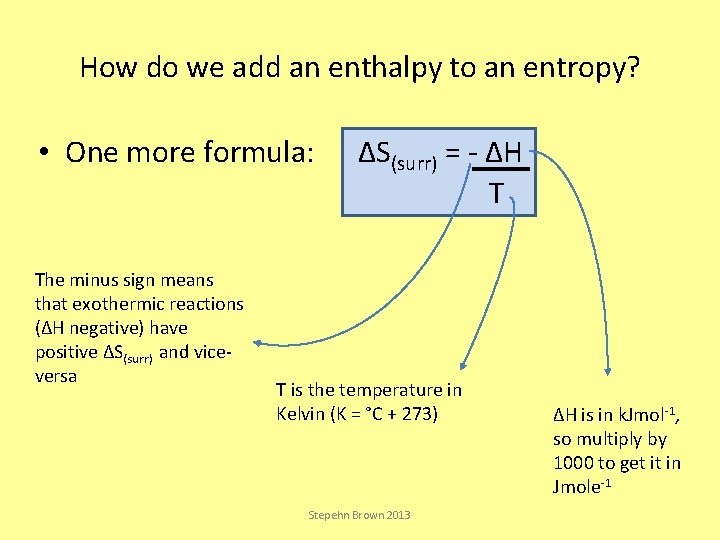
How do we add an enthalpy to an entropy? • One more formula: The minus sign means that exothermic reactions (ΔH negative) have positive ΔS(surr) and viceversa ΔS(surr) = - ΔH T T is the temperature in Kelvin (K = °C + 273) Stepehn Brown 2013 ΔH is in k. Jmol-1, so multiply by 1000 to get it in Jmole-1
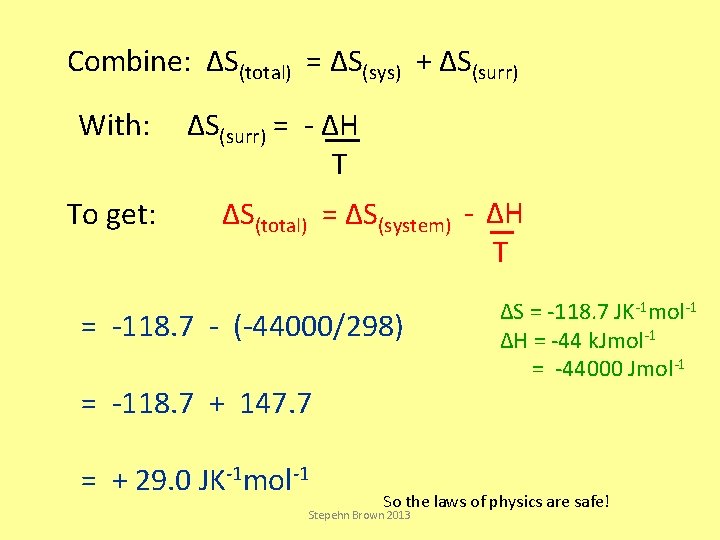
Combine: ΔS(total) = ΔS(sys) + ΔS(surr) With: To get: ΔS(surr) = - ΔH T ΔS(total) = ΔS(system) - ΔH T = -118. 7 - (-44000/298) = -118. 7 + 147. 7 = + 29. 0 JK-1 mol-1 ΔS = -118. 7 JK-1 mol-1 ΔH = -44 k. Jmol-1 = -44000 Jmol-1 So the laws of physics are safe! Stepehn Brown 2013
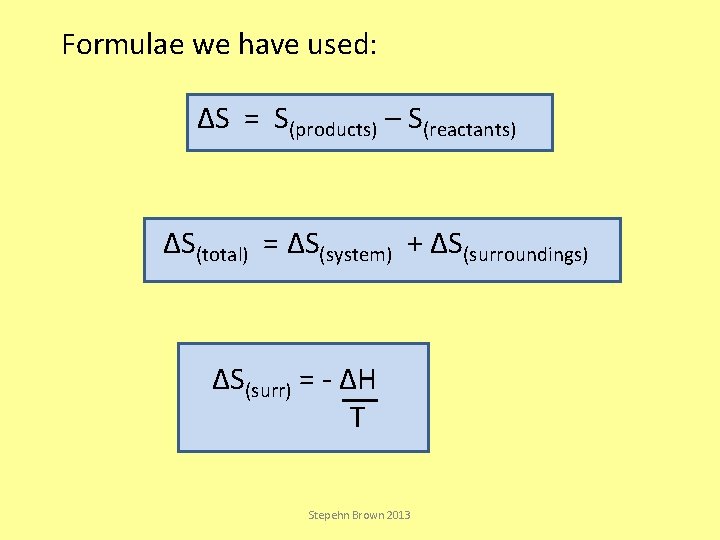
Formulae we have used: ΔS = S(products) – S(reactants) ΔS(total) = ΔS(system) + ΔS(surroundings) ΔS(surr) = - ΔH T Stepehn Brown 2013
Explain entropy changes in a qualitative manner, interpreting entropy as a measure of the number of ways that molecules and their associated energy quanta can be arranged Calculate the entropy change of a reaction given the entropies of reactants and products Recall the expressions ΔStot = ΔSsys + ΔSsurr and ΔSsurr = -ΔH/T Be able to perform calculation using these expressions Explain the tendency for a reaction to occur in terms of the sign of ΔStot Stepehn Brown 2013
- Slides: 12