Entrapment Method For Enzyme Immobilization Mr Nilesh Gaikar

Entrapment: Method For Enzyme Immobilization Mr. Nilesh Gaikar, Asst. Professor Department: School of Pharmacy Subject: Pharmaceutical Biotechnology Semester: V Teaching Aids Service by KRRC Information Section

ENTRAPMENT ØEnzymes are physically entrapped inside a matrix. ØBonds involved may be covalent or non covalent ØMatrix used will be water soluble polymer ØGenerally entrapment does not involve a chemical or physical/chemical reaction directly with enzyme molecules. ØPhysical adsorption on active carbon particles and ionic adsorption on ion exchange resins are important for enzyme immobilisation

ENTRAPMENT ØForm and nature of matrix varies ØPore size of matrix is adjusted to prevent the loss of enzyme ØPossibility of leakage of low molecular weight enzymes ØAgar and carragrenan have large pore sizes ØPore size can be adjusted with the concentration of the polymer

Methods of Entrapment üInclusion in gels: enzymes are trapped in gels üInclusion in microcapsules: enzymes entrapped in micromolecules formed by monomer mixture such as calcium alginate, polyamine etc. .

§Polyacrylamide is the most widely used matrix for entrapping enzymes among the gels. It is non-ionic §The properties of the enzymes are modified minimum in the presence of the gel matrix. § At the same time, the diffusion of the charged substrate and products is not affected. §Dimethylaminopropionitrile is the polymerisation initiator that is highly toxic §it must be handled with great care. §Calcium alginate does not depend on the formation of more permanent covalent bonds between polymer chains §calcium ions cross-link the polymer molecules § beads formed in mild conditions, enzyme activity over 80% can be achieved §As calcium ions can be exchanged for sodium ions, similarly they can also be displaced by other ions §care must be taken to ensure that the substrate solution does not contain high concentrations of such ions that disintegrate the gel.

Entrapment method difficulties : (i) leakage due to wide pore size of the gel [for example, agar, carrageenan, etc. have large « 10 microns)], (ii) reduced substrate accessibility to the enzyme (iii) some loss of activity due to the free radicals produced during polymerization of the gel. §entrapment of enzymes has been widely used for sensing application §Cell entrapment is used for industrial production of amino acids such as Laspartic acid, L-malic acid etc.
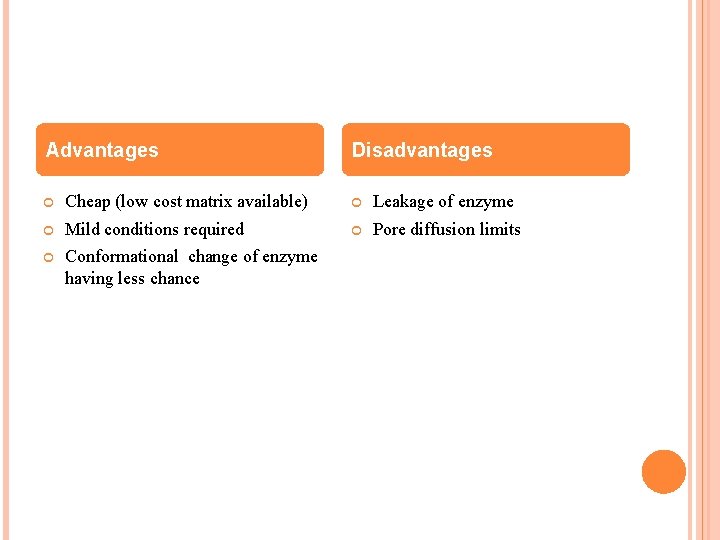
Advantages Disadvantages Cheap (low cost matrix available) Leakage of enzyme Mild conditions required Pore diffusion limits Conformational change of enzyme having less chance

ENCAPSULATION Øenclosing of a droplet of solution of ezymein semipermeable membrane capsule. Ømembrane may be polymeric, lipoidal, lipoprotein basednon-ionic in nature. Ø capsule is made up of cellulose nitrate and nylon Øeffectiveness largely depends on the stability of enzyme
ØImmobilization of enzymes and mammalian cells ØPancreatic cells grown in cultures immobilized by encapsulation Three different ways of encapsulation on basis of ØSpecific membrane reactors ØFormation of emulation ØStabilisation of emulation to form microcapsule üEncapsulation of enzyme in mesoporous silica spheres via immobilization followed by assembling an organic/inorganic nanocomposite shell on the particle surface leads to high loading enzyme activity and stability protection proteolysis from

Advantage Øcheap and simple ØLarge quantities of enzyme can be immobilized Disadvantages §Pore size limitation §Only small substrate molecule is able to cross the membrane
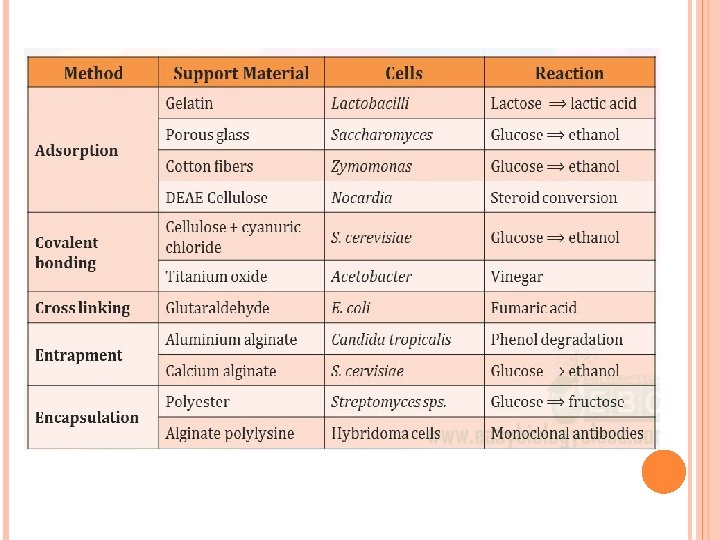
- Slides: 11