Enthalpy International Junior Science Olympiad IJSO Dr YuSan






![Solution: From the first reaction: 74. 85 = 0 + Hof [Cl (aq)] ( Solution: From the first reaction: 74. 85 = 0 + Hof [Cl (aq)] (](https://slidetodoc.com/presentation_image/5ef8a2b069bd24ed6fe09b0e9fc7f2a1/image-25.jpg)
- Slides: 27

Enthalpy International Junior Science Olympiad (IJSO) Dr. Yu-San Cheung yscheung@cuhk. edu. hk Department of Chemistry The Chinese University of Hong Kong 1

Enthalpy(焓) Enthalpy (H) is the heat content of a substance. It can also be considered as the total energy of a substance. When a physical process or chemical reaction occurs, heat is usually given out or absorbed from the surroundings. e. g. , ice melting, condensation of water vapor, burning of coal. 2

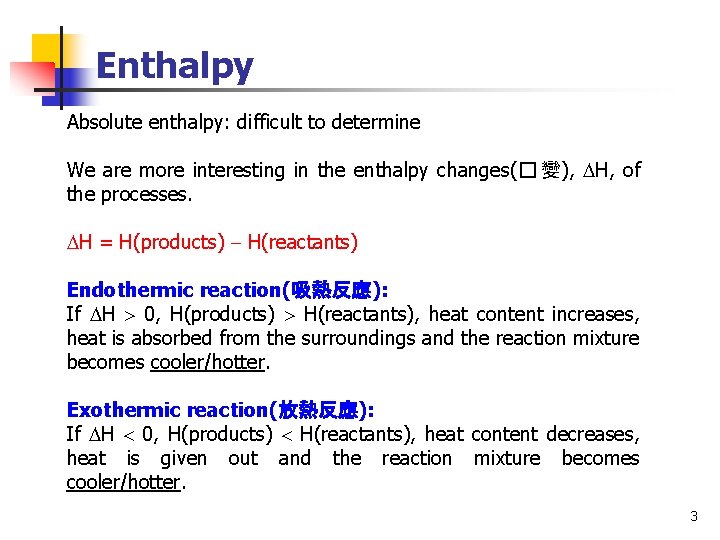
Enthalpy Absolute enthalpy: difficult to determine We are more interesting in the enthalpy changes(� 變), H, of the processes. H = H(products) H(reactants) Endothermic reaction(吸熱反應): If H 0, H(products) H(reactants), heat content increases, heat is absorbed from the surroundings and the reaction mixture becomes cooler/hotter. Exothermic reaction(放熱反應): If H 0, H(products) H(reactants), heat content decreases, heat is given out and the reaction mixture becomes cooler/hotter. 3

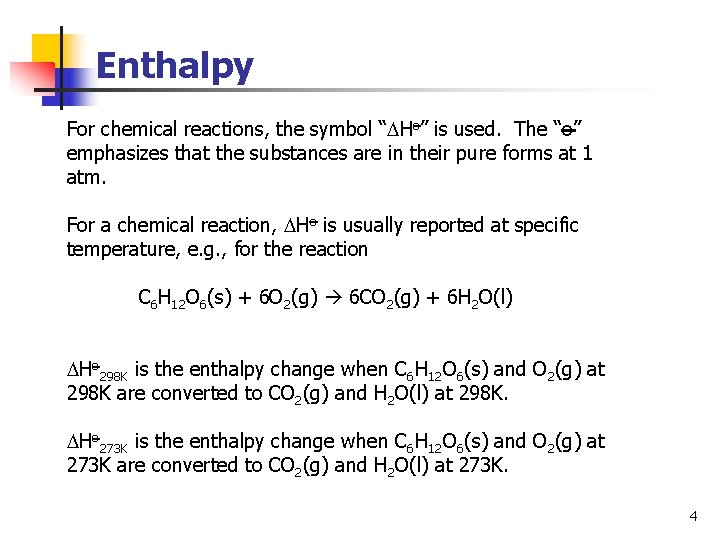
Enthalpy For chemical reactions, the symbol “ Ho” is used. The “o” emphasizes that the substances are in their pure forms at 1 atm. For a chemical reaction, Ho is usually reported at specific temperature, e. g. , for the reaction C 6 H 12 O 6(s) + 6 O 2(g) 6 CO 2(g) + 6 H 2 O(l) Ho 298 K is the enthalpy change when C 6 H 12 O 6(s) and O 2(g) at 298 K are converted to CO 2(g) and H 2 O(l) at 298 K. Ho 273 K is the enthalpy change when C 6 H 12 O 6(s) and O 2(g) at 273 K are converted to CO 2(g) and H 2 O(l) at 273 K. 4

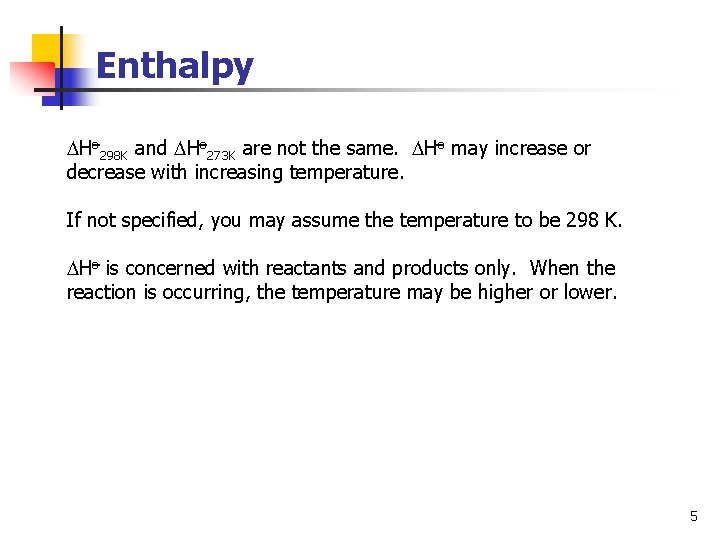
Enthalpy Ho 298 K and Ho 273 K are not the same. Ho may increase or decrease with increasing temperature. If not specified, you may assume the temperature to be 298 K. Ho is concerned with reactants and products only. When the reaction is occurring, the temperature may be higher or lower. 5

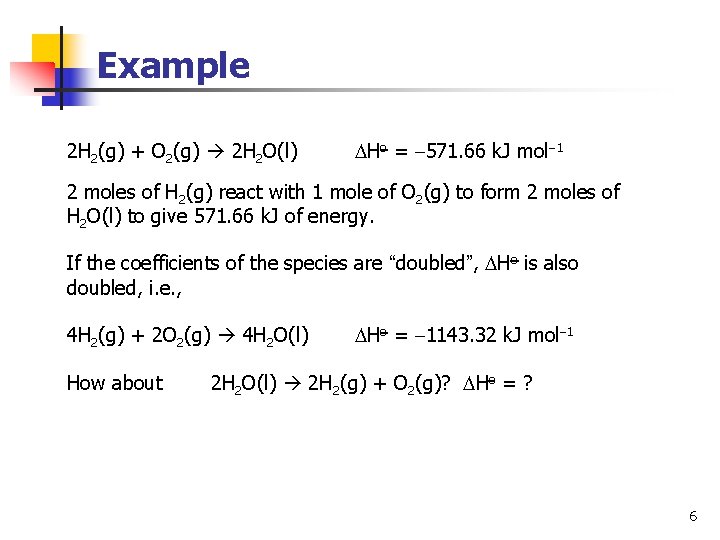
Example 2 H 2(g) + O 2(g) 2 H 2 O(l) Ho = 571. 66 k. J mol 1 2 moles of H 2(g) react with 1 mole of O 2(g) to form 2 moles of H 2 O(l) to give 571. 66 k. J of energy. If the coefficients of the species are “doubled”, Ho is also doubled, i. e. , 4 H 2(g) + 2 O 2(g) 4 H 2 O(l) How about Ho = 1143. 32 k. J mol 1 2 H 2 O(l) 2 H 2(g) + O 2(g)? Ho = ? 6

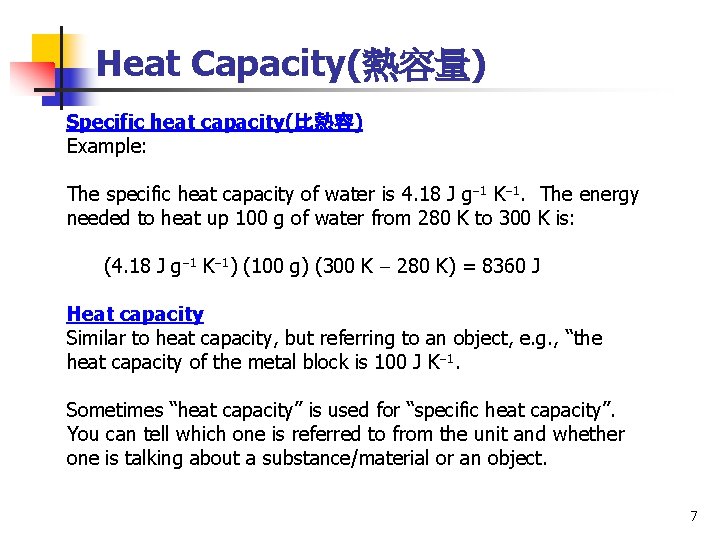
Heat Capacity(熱容量) Specific heat capacity(比熱容) Example: The specific heat capacity of water is 4. 18 J g 1 K 1. The energy needed to heat up 100 g of water from 280 K to 300 K is: (4. 18 J g 1 K 1) (100 g) (300 K 280 K) = 8360 J Heat capacity Similar to heat capacity, but referring to an object, e. g. , “the heat capacity of the metal block is 100 J K 1. Sometimes “heat capacity” is used for “specific heat capacity”. You can tell which one is referred to from the unit and whether one is talking about a substance/material or an object. 7

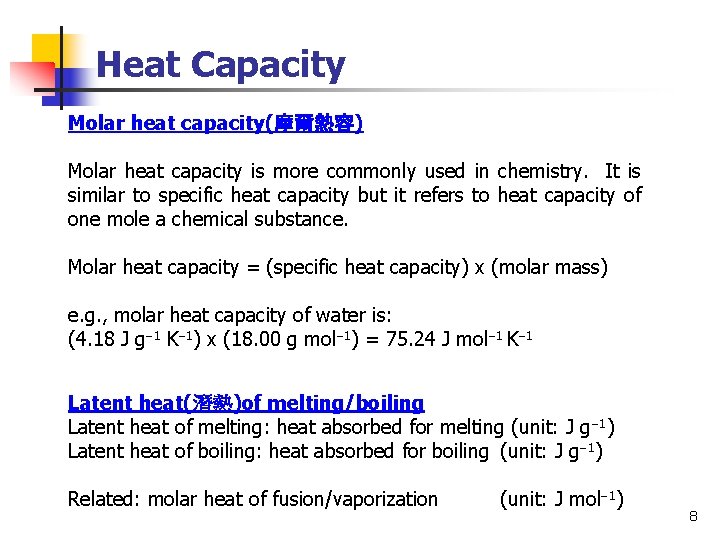
Heat Capacity Molar heat capacity(摩爾熱容) Molar heat capacity is more commonly used in chemistry. It is similar to specific heat capacity but it refers to heat capacity of one mole a chemical substance. Molar heat capacity = (specific heat capacity) x (molar mass) e. g. , molar heat capacity of water is: (4. 18 J g 1 K 1) x (18. 00 g mol 1) = 75. 24 J mol 1 K 1 Latent heat(潛熱)of melting/boiling Latent heat of melting: heat absorbed for melting (unit: J g 1) Latent heat of boiling: heat absorbed for boiling (unit: J g 1) Related: molar heat of fusion/vaporization (unit: J mol 1) 8

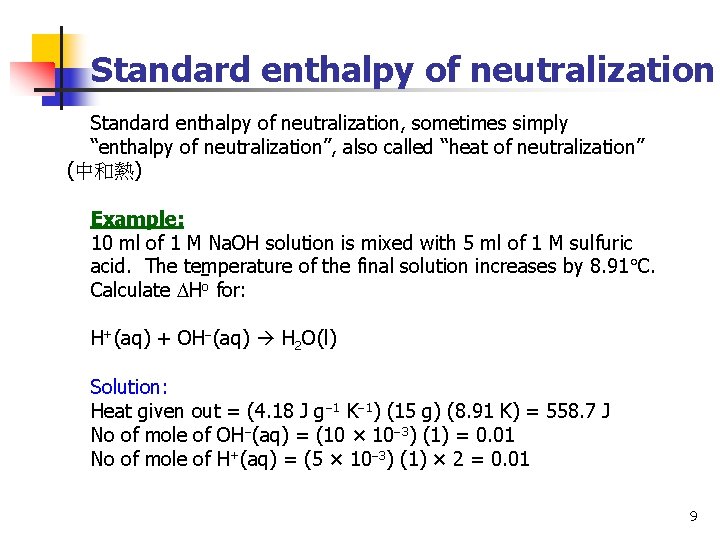
Standard enthalpy of neutralization, sometimes simply “enthalpy of neutralization”, also called “heat of neutralization” (中和熱) Example: 10 ml of 1 M Na. OH solution is mixed with 5 ml of 1 M sulfuric acid. The temperature of the final solution increases by 8. 91 C. Calculate Ho for: H+(aq) + OH (aq) H 2 O(l) Solution: Heat given out = (4. 18 J g 1 K 1) (15 g) (8. 91 K) = 558. 7 J No of mole of OH (aq) = (10 × 10 3) (1) = 0. 01 No of mole of H+(aq) = (5 × 10 3) (1) × 2 = 0. 01 9

Standard enthalpy of neutralization Therefore, if 1 mole of H+(aq) reacts with 1 mole of OH (aq), 55870 J (or 55. 87 k. J) is given out. And because heat is given out, H is negative. Therefore, Ho = 55. 87 k. J mol 1 Note that in the above mixing, Na. OH solution is a strong alkali and sulfuric acid is a strong acid. If 1 L of 1 M Na. OH solution is mixed with 1 L of 1 M acetic acid, the heat given out is smaller/larger than 55. 87 k. J. 10

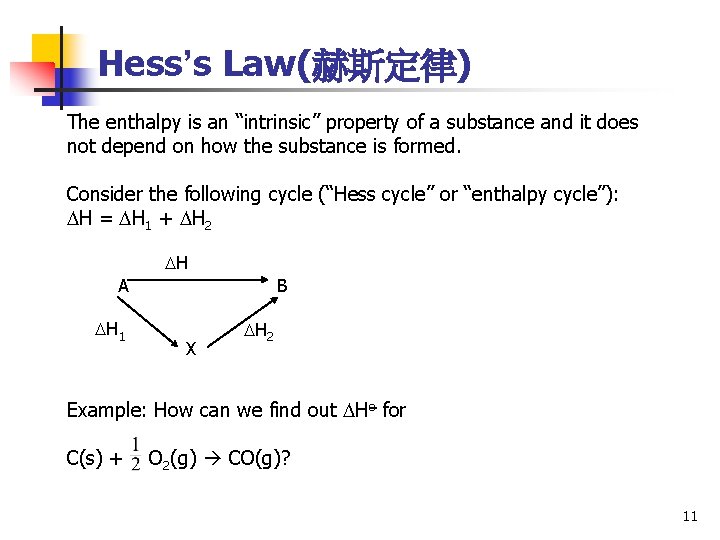
Hess’s Law(赫斯定律) The enthalpy is an “intrinsic” property of a substance and it does not depend on how the substance is formed. Consider the following cycle (“Hess cycle” or “enthalpy cycle”): H = H 1 + H 2 H A H 1 B X H 2 Example: How can we find out Ho for C(s) + O 2(g) CO(g)? 11

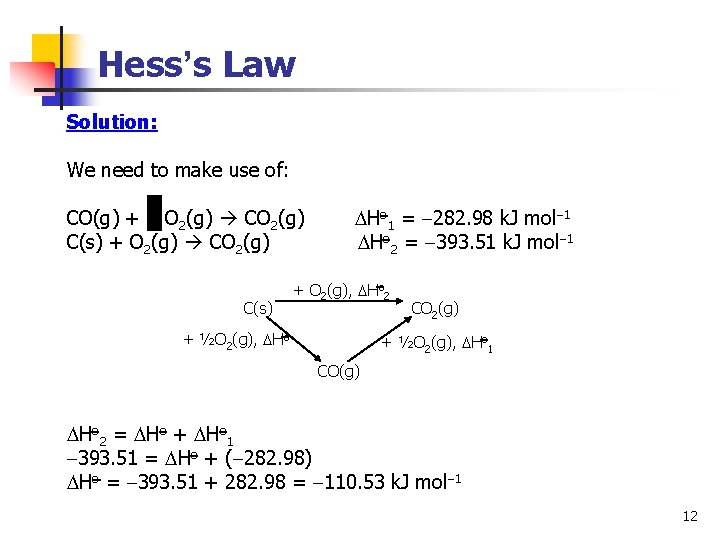
Hess’s Law Solution: We need to make use of: CO(g) + O 2(g) CO 2(g) Ho 1 = 282. 98 k. J mol 1 C(s) + O 2(g) CO 2(g) Ho 2 = 393. 51 k. J mol 1 C(s) + O 2(g), Ho 2 + ½O 2(g), Ho CO 2(g) + ½O 2(g), Ho 1 CO(g) Ho 2 = Ho + Ho 1 393. 51 = Ho + ( 282. 98) Ho = 393. 51 + 282. 98 = 110. 53 k. J mol 1 12

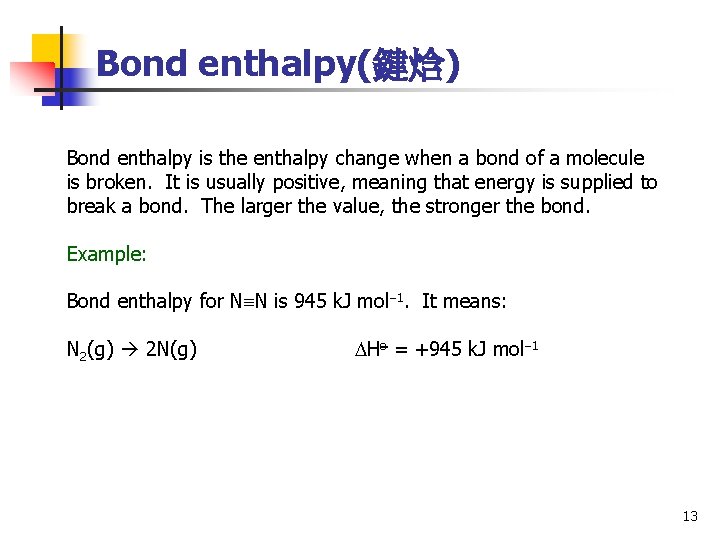
Bond enthalpy(鍵焓) Bond enthalpy is the enthalpy change when a bond of a molecule is broken. It is usually positive, meaning that energy is supplied to break a bond. The larger the value, the stronger the bond. Example: Bond enthalpy for N N is 945 k. J mol 1. It means: N 2(g) 2 N(g) Ho = +945 k. J mol 1 13

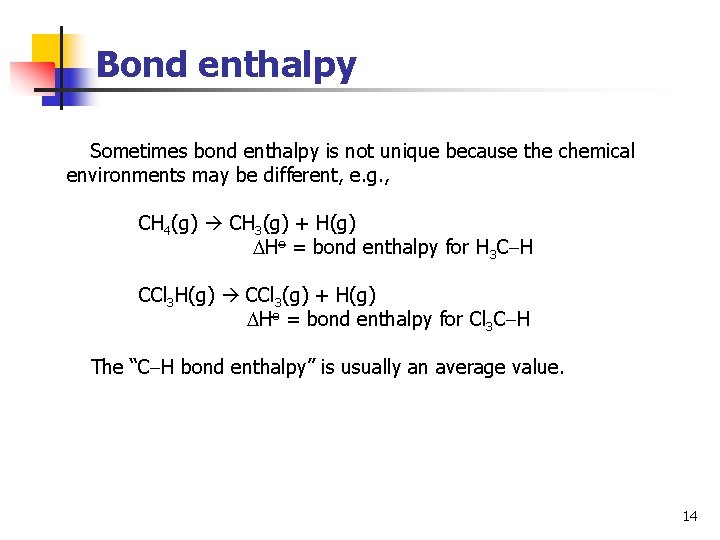
Bond enthalpy Sometimes bond enthalpy is not unique because the chemical environments may be different, e. g. , CH 4(g) CH 3(g) + H(g) Ho = bond enthalpy for H 3 C H CCl 3 H(g) CCl 3(g) + H(g) Ho = bond enthalpy for Cl 3 C H The “C H bond enthalpy” is usually an average value. 14

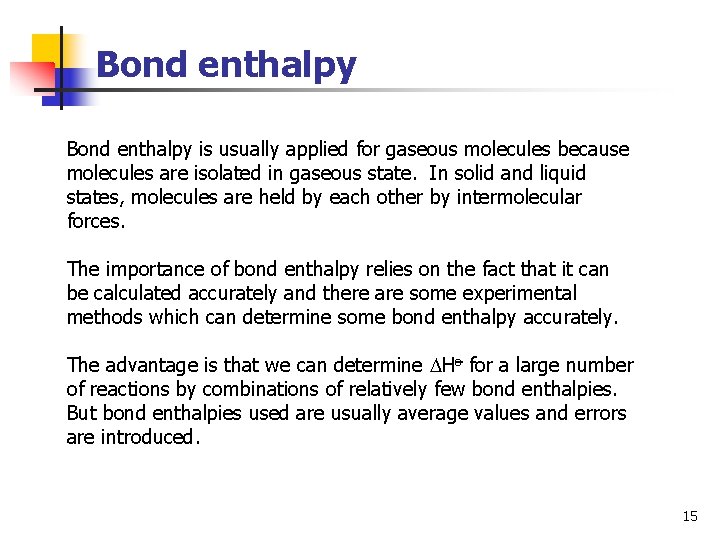
Bond enthalpy is usually applied for gaseous molecules because molecules are isolated in gaseous state. In solid and liquid states, molecules are held by each other by intermolecular forces. The importance of bond enthalpy relies on the fact that it can be calculated accurately and there are some experimental methods which can determine some bond enthalpy accurately. The advantage is that we can determine Ho for a large number of reactions by combinations of relatively few bond enthalpies. But bond enthalpies used are usually average values and errors are introduced. 15

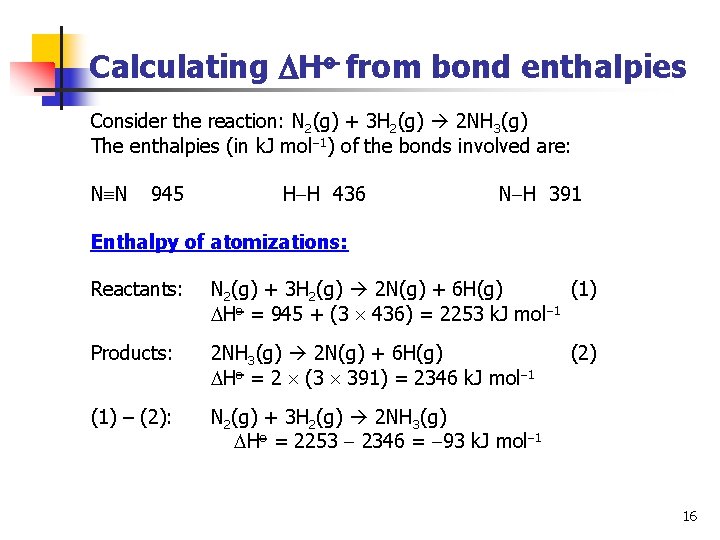
Calculating Ho from bond enthalpies Consider the reaction: N 2(g) + 3 H 2(g) 2 NH 3(g) The enthalpies (in k. J mol 1) of the bonds involved are: N N 945 H H 436 N H 391 Enthalpy of atomizations: Reactants: N 2(g) + 3 H 2(g) 2 N(g) + 6 H(g) (1) Ho = 945 + (3 436) = 2253 k. J mol 1 Products: 2 NH 3(g) 2 N(g) + 6 H(g) Ho = 2 (3 391) = 2346 k. J mol 1 (2) (1) – (2): N 2(g) + 3 H 2(g) 2 NH 3(g) Ho = 2253 2346 = 93 k. J mol 1 16

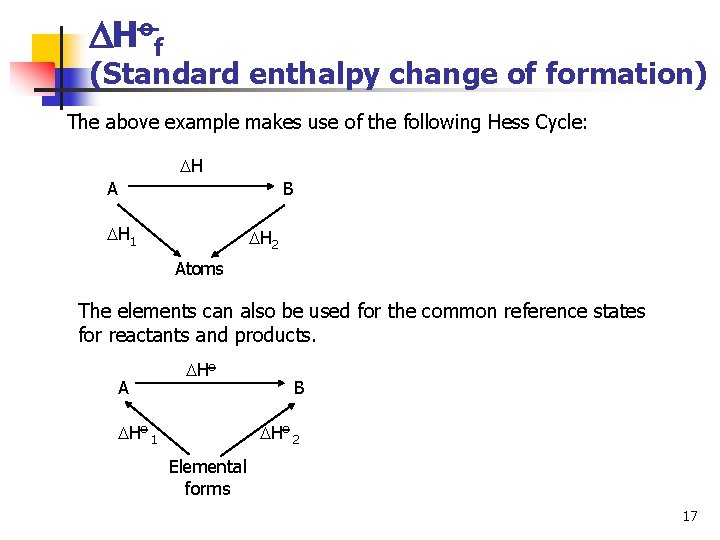
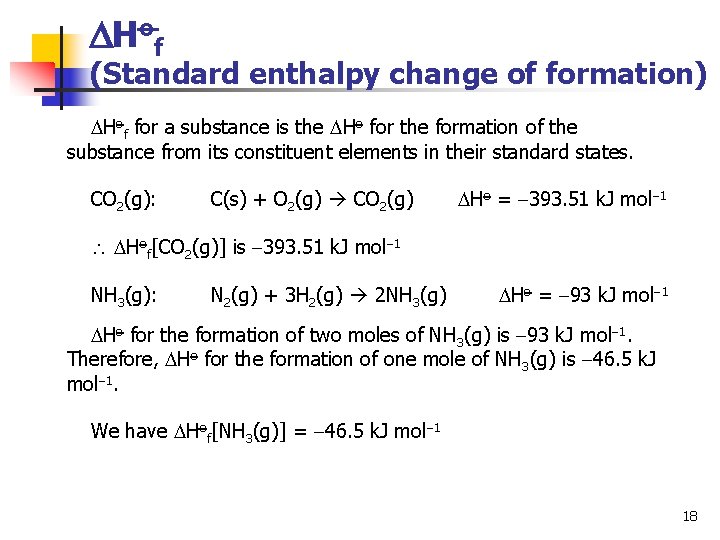
Hof (Standard enthalpy change of formation) The above example makes use of the following Hess Cycle: H A B H 1 H 2 Atoms The elements can also be used for the common reference states for reactants and products. A Ho 1 B Ho 2 Elemental forms 17

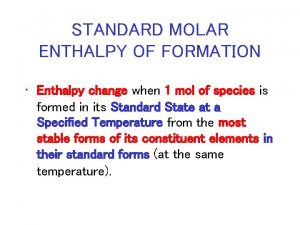
Hof (Standard enthalpy change of formation) Hof for a substance is the Ho for the formation of the substance from its constituent elements in their standard states. CO 2(g): C(s) + O 2(g) CO 2(g) Ho = 393. 51 k. J mol 1 Hof[CO 2(g)] is 393. 51 k. J mol 1 NH 3(g): N 2(g) + 3 H 2(g) 2 NH 3(g) Ho = 93 k. J mol 1 Ho for the formation of two moles of NH 3(g) is 93 k. J mol 1. Therefore, Ho for the formation of one mole of NH 3(g) is 46. 5 k. J mol 1. We have Hof[NH 3(g)] = 46. 5 k. J mol 1 18

Remarks: • If an element exists in different forms, the most stable form is chosen for the standard states. Carbon: graphite, NOT diamond Oxygen: O 2, NOT O 3 • Hof for the elements at the standard states is zero. • Hof for aqueous ions: (a) Compounds come with both cations and anions. It is impossible to determine Hof for a single cation or anion. Therefore, Hof for H+(aq) is set to zero (by human). 19

Remarks: (b) Strictly speaking, Hof for aqueous ions depends on their concentrations. For example, heat changes for dissolving one mole of Na. Cl in 1 L of water and in 10000 L of water are different. We usually assume the concentration is indefinitely low. • Hof values for many compounds are tabulated. If the values for the reactants and products of a reaction can be found, we can determine Ho for this reaction. 20

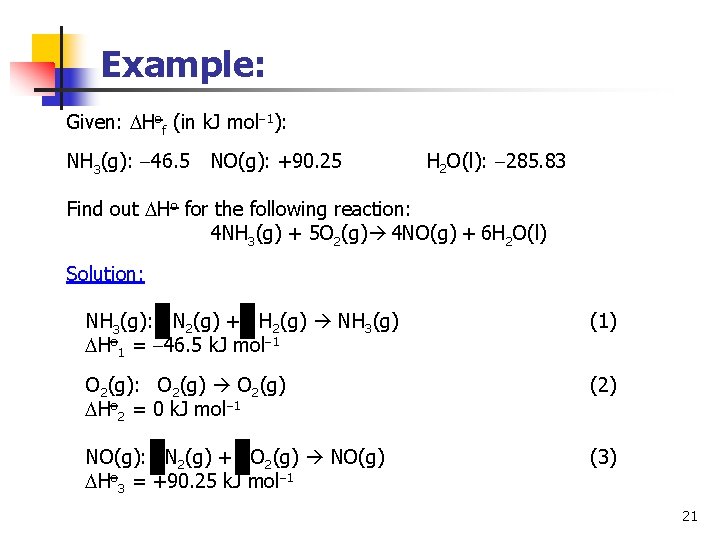
Example: Given: Hof (in k. J mol 1): NH 3(g): 46. 5 NO(g): +90. 25 H 2 O(l): 285. 83 Find out Ho for the following reaction: 4 NH 3(g) + 5 O 2(g) 4 NO(g) + 6 H 2 O(l) Solution: NH 3(g): N 2(g) + H 2(g) NH 3(g) Ho 1 = 46. 5 k. J mol 1 (1) O 2(g): O 2(g) Ho 2 = 0 k. J mol 1 (2) NO(g): N 2(g) + O 2(g) NO(g) Ho 3 = +90. 25 k. J mol 1 (3) 21

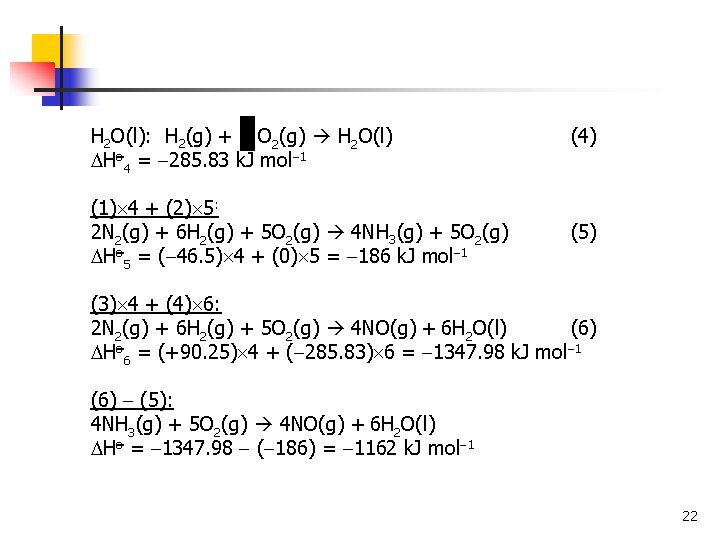
H 2 O(l): H 2(g) + O 2(g) H 2 O(l) Ho 4 = 285. 83 k. J mol 1 (1) 4 + (2) 5: 2 N 2(g) + 6 H 2(g) + 5 O 2(g) 4 NH 3(g) + 5 O 2(g) Ho 5 = ( 46. 5) 4 + (0) 5 = 186 k. J mol 1 (4) (5) (3) 4 + (4) 6: 2 N 2(g) + 6 H 2(g) + 5 O 2(g) 4 NO(g) + 6 H 2 O(l) (6) Ho 6 = (+90. 25) 4 + ( 285. 83) 6 = 1347. 98 k. J mol 1 (6) (5): 4 NH 3(g) + 5 O 2(g) 4 NO(g) + 6 H 2 O(l) Ho = 1347. 98 ( 186) = 1162 k. J mol 1 22

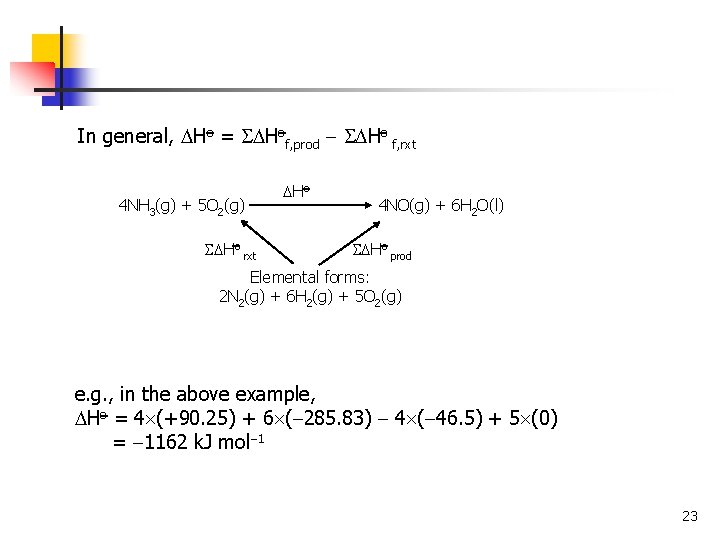
In general, Ho = Hof, prod Ho f, rxt 4 NH 3(g) + 5 O 2(g) Ho rxt Ho 4 NO(g) + 6 H 2 O(l) Ho prod Elemental forms: 2 N 2(g) + 6 H 2(g) + 5 O 2(g) e. g. , in the above example, Ho = 4 (+90. 25) + 6 ( 285. 83) 4 ( 46. 5) + 5 (0) = 1162 k. J mol 1 23

Example: Calculate Hof for Cl (aq) and Na+(aq) using the following Ho: HCl(g) H+(aq) + Cl (aq) Na. Cl(s) Na+(aq) + Cl (aq) Ho = 74. 85 k. J mol 1 Ho = +3. 87 k. J mol 1 Also given Hof values (in k. J mol 1): HCl(g): 92. 31 Na. Cl(s): 411. 15 24
![Solution From the first reaction 74 85 0 Hof Cl aq Solution: From the first reaction: 74. 85 = 0 + Hof [Cl (aq)] (](https://slidetodoc.com/presentation_image/5ef8a2b069bd24ed6fe09b0e9fc7f2a1/image-25.jpg)
Solution: From the first reaction: 74. 85 = 0 + Hof [Cl (aq)] ( 92. 31) Hof [Cl (aq)] = 167. 16 k. J mol 1 From the second reaction: +3. 87 = Hof [Na+(aq)] + ( 167. 16) ( 411. 15) Hof [Na+(aq)] = 240. 12 k. J mol 1 25

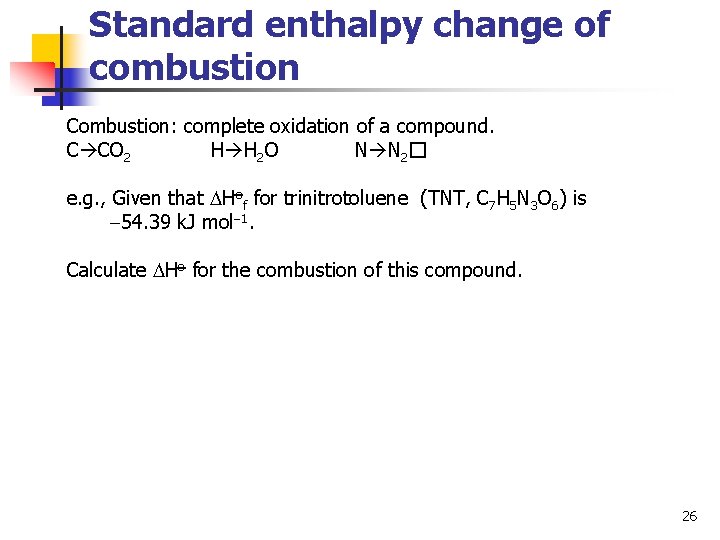
Standard enthalpy change of combustion Combustion: complete oxidation of a compound. C CO 2 H H 2 O N N 2� e. g. , Given that Hof for trinitrotoluene (TNT, C 7 H 5 N 3 O 6) is 54. 39 k. J mol 1. Calculate Ho for the combustion of this compound. 26

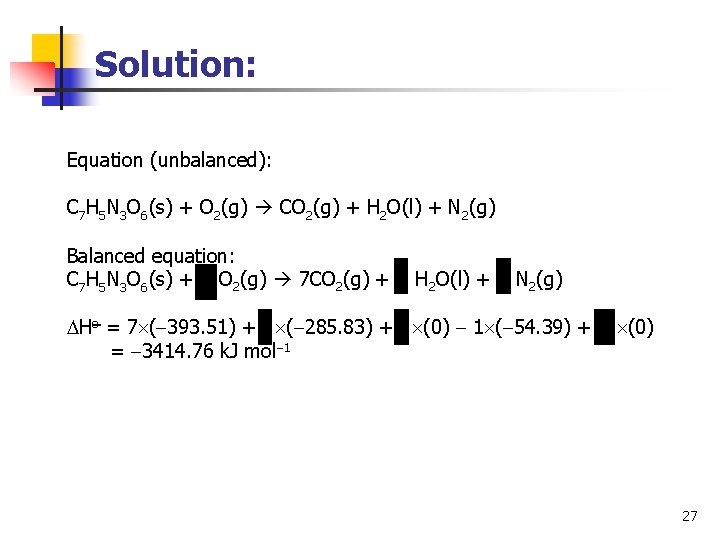
Solution: Equation (unbalanced): C 7 H 5 N 3 O 6(s) + O 2(g) CO 2(g) + H 2 O(l) + N 2(g) Balanced equation: C 7 H 5 N 3 O 6(s) + O 2(g) 7 CO 2(g) + H 2 O(l) + N 2(g) Ho = 7 ( 393. 51) + ( 285. 83) + (0) 1 ( 54. 39) + (0) = 3414. 76 k. J mol 1 27
 Junior science olympiad
Junior science olympiad Ijso 2011
Ijso 2011 Anatomy science olympiad
Anatomy science olympiad Detector building science olympiad cheat sheet
Detector building science olympiad cheat sheet Science olympiad fossils
Science olympiad fossils Science olympiad forensics cheat sheet
Science olympiad forensics cheat sheet Science olympiad protein modeling
Science olympiad protein modeling Ornithology practice test div c
Ornithology practice test div c Science olympiad solar system
Science olympiad solar system Microbe mission science olympiad
Microbe mission science olympiad Green generation science olympiad
Green generation science olympiad Gravity vehicle
Gravity vehicle Elementary science olympiad georgia
Elementary science olympiad georgia Can't judge a powder
Can't judge a powder Science olympiad summer institute
Science olympiad summer institute Science olympiad wright stuff kit
Science olympiad wright stuff kit Westview science olympiad
Westview science olympiad Machines science olympiad
Machines science olympiad Science olympiad boomilever
Science olympiad boomilever Environmental chemistry science olympiad
Environmental chemistry science olympiad Ping pong parachute design
Ping pong parachute design State bird of washington
State bird of washington Brookwood science olympiad invitational
Brookwood science olympiad invitational Deep blue sea science olympiad
Deep blue sea science olympiad Richard ito
Richard ito Ornithology science olympiad cheat sheet
Ornithology science olympiad cheat sheet Science olympiad herpetology
Science olympiad herpetology Astronomy science olympiad
Astronomy science olympiad