Enthalpy Exothermic and Endothermic Processes Direction of heat






- Slides: 26

Enthalpy, Exothermic and Endothermic Processes Direction of heat flow in chemical reactions

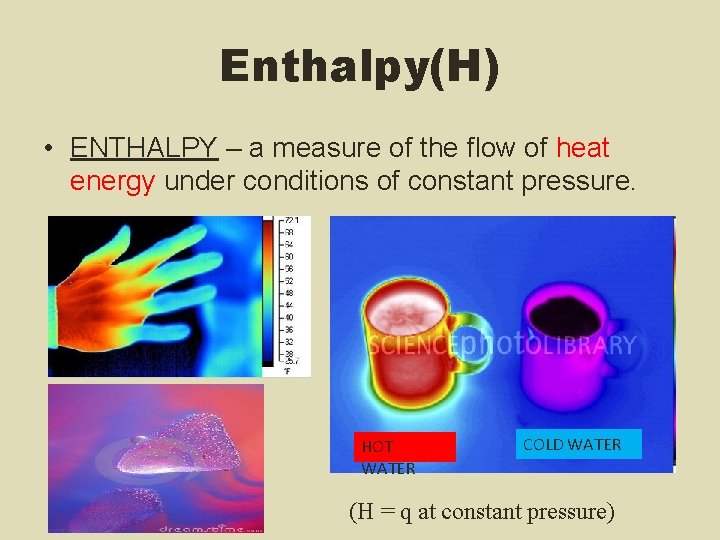
Enthalpy(H) • ENTHALPY – a measure of the flow of heat energy under conditions of constant pressure. HOT WATER COLD WATER (H = q at constant pressure)

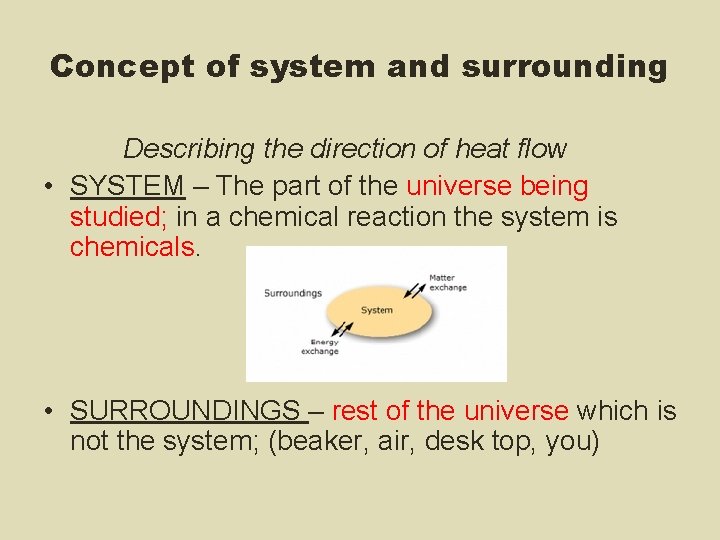
Concept of system and surrounding Describing the direction of heat flow • SYSTEM – The part of the universe being studied; in a chemical reaction the system is chemicals. • SURROUNDINGS – rest of the universe which is not the system; (beaker, air, desk top, you)

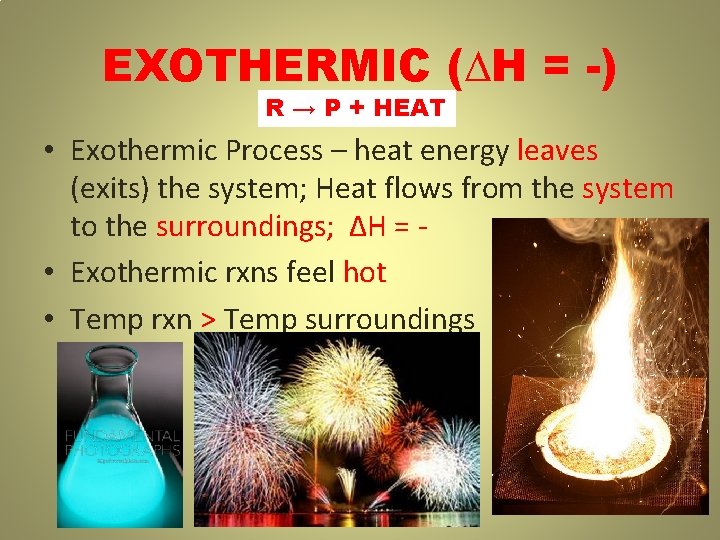
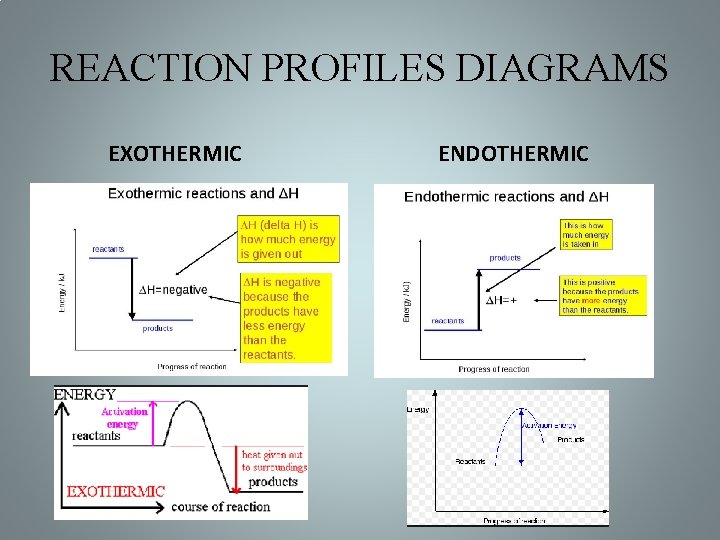
EXOTHERMIC (∆H = -) R → P + HEAT • Exothermic Process – heat energy leaves (exits) the system; Heat flows from the system to the surroundings; ∆H = • Exothermic rxns feel hot • Temp rxn > Temp surroundings

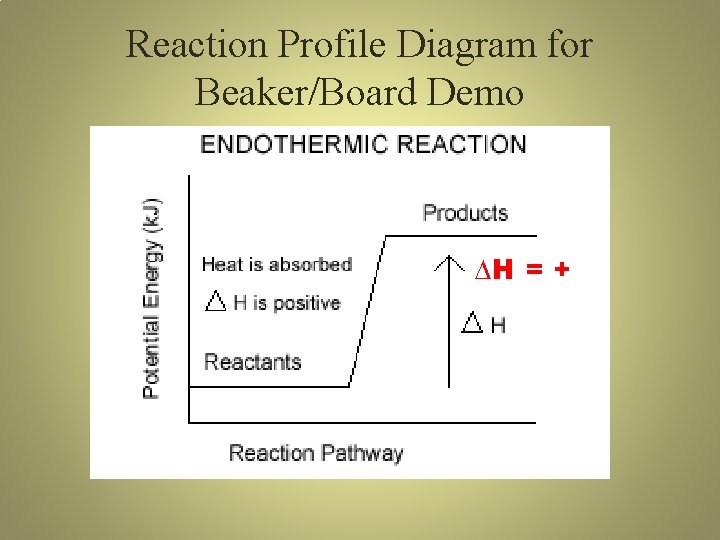
Endothermic Reaction R + HEAT → P • Endothermic process – heat energy flows into (enters) system from surroundings; ∆H = + • Endothermic reactions feel cold • Temp rxn < Temp surr

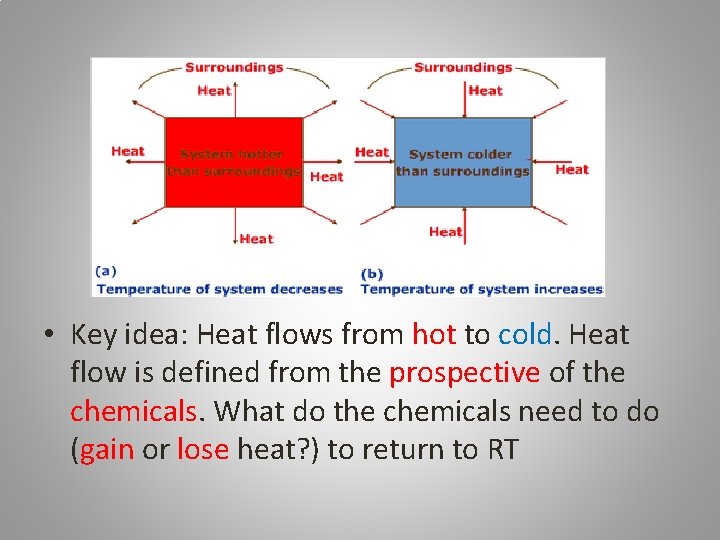
• Key idea: Heat flows from hot to cold. Heat flow is defined from the prospective of the chemicals. What do the chemicals need to do (gain or lose heat? ) to return to RT

REACTION PROFILES DIAGRAMS EXOTHERMIC ENDOTHERMIC

Demonstration of NH 4 Cl in water Chemicals (NH 4 Cl + H 2 O) = System Beaker, desk top, air, you = Surroundings Feels: COLD Temp chem < T Surrounding Heat flows from Surroundings to System ENDOTHERMIC ; ∆H = +

Demonstration of Ca. Cl 2 in water Chemicals (Ca. Cl 2 + H 2 O) = System Beaker, desktop, air, you = Surroundings Feels: Hot Temp chem > Temp Surr Heat flows from system → Surroundings Exothermic ; ∆H = -

Hand-Warmer Demonstration Super-saturated solution Contains more sodium acetate dissolved in water volume than is stable at room temperature. Prepared by heating a saturated solution to high temperature and allowing solution to cool slowly. Video link seed crystal Fun with sodium Ac video

Reaction of Potassium Permanganate and Glycerin Initial Observations Final observations • Thick viscous liquid is dropped on pile of dark purple/black crystals • Bright purple, flames, smoke, very hot Video link

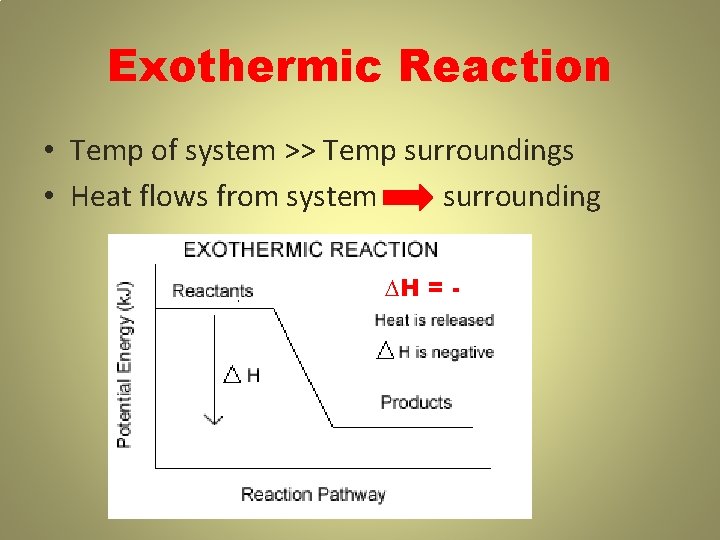
Exothermic Reaction • Temp of system >> Temp surroundings • Heat flows from system surrounding ∆H = -

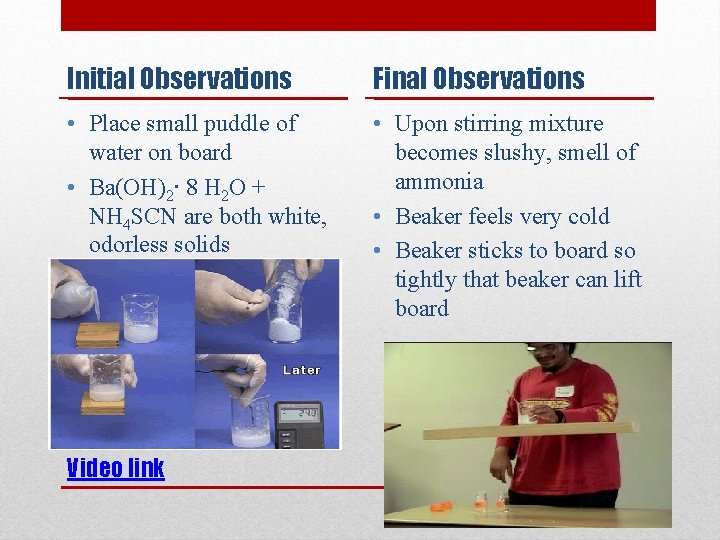
Initial Observations Final Observations • Place small puddle of water on board • Ba(OH)2∙ 8 H 2 O + NH 4 SCN are both white, odorless solids • Upon stirring mixture becomes slushy, smell of ammonia • Beaker feels very cold • Beaker sticks to board so tightly that beaker can lift board Video link

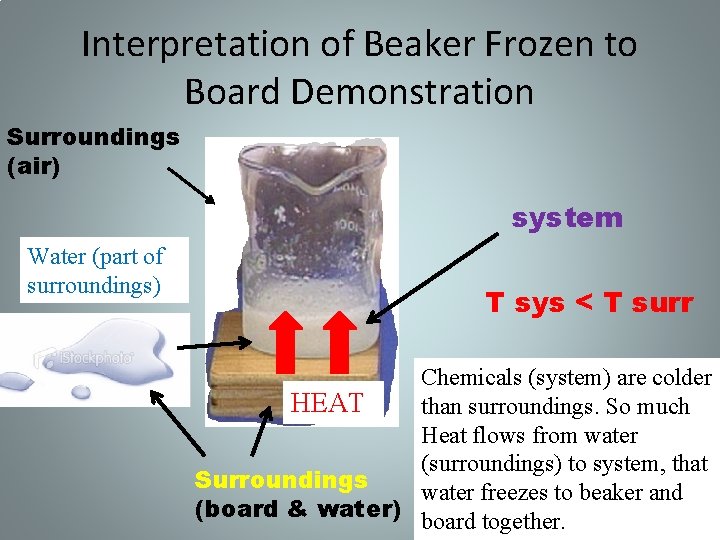
Interpretation of Beaker Frozen to Board Demonstration Surroundings (air) system Water (part of surroundings) T sys < T surr Chemicals (system) are colder HEAT than surroundings. So much Heat flows from water (surroundings) to system, that Surroundings water freezes to beaker and (board & water) board together.

Reaction Profile Diagram for Beaker/Board Demo ∆H = +

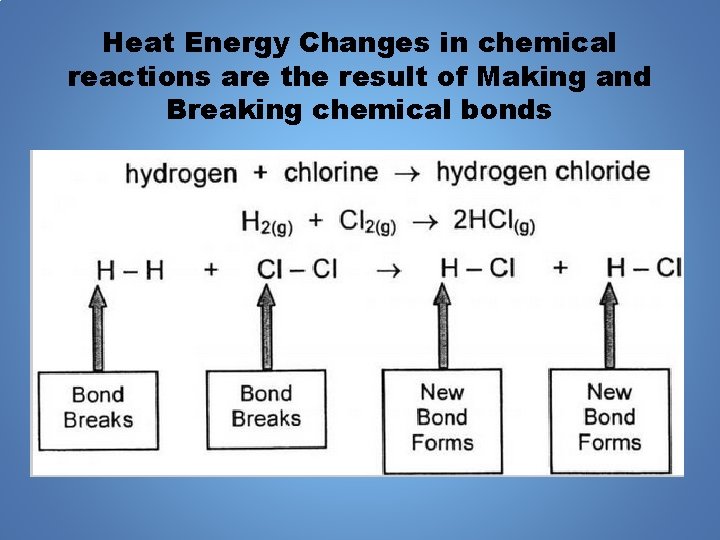
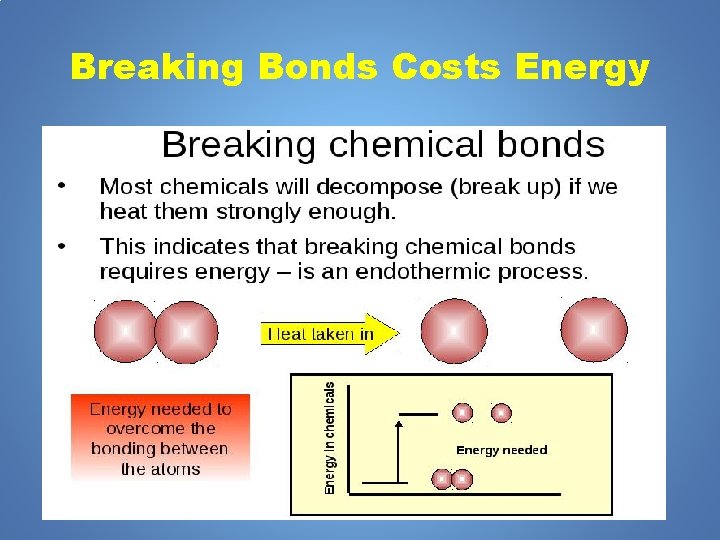
Heat Energy Changes in chemical reactions are the result of Making and Breaking chemical bonds

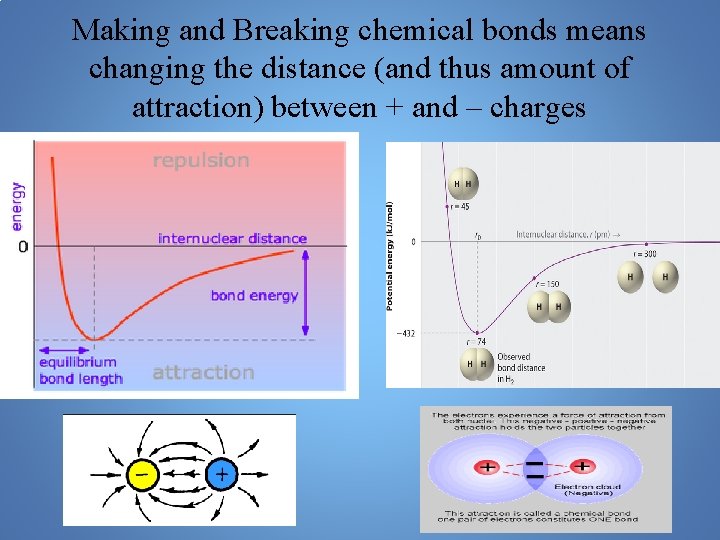
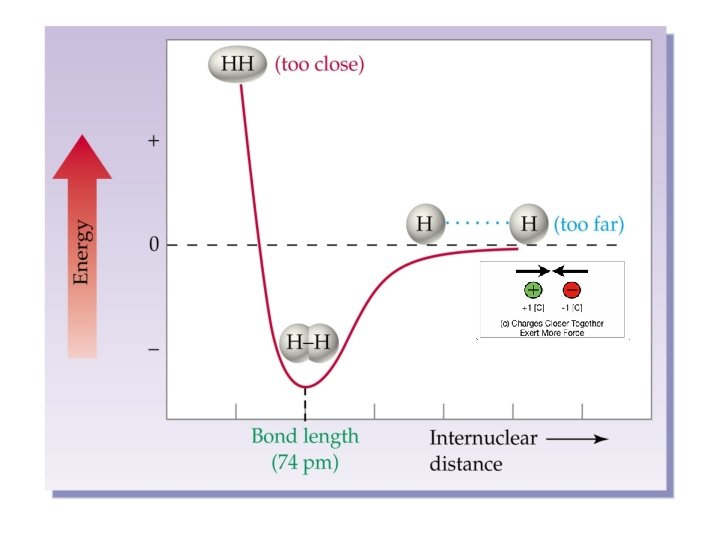
Making and Breaking chemical bonds means changing the distance (and thus amount of attraction) between + and – charges


Breaking Bonds Costs Energy

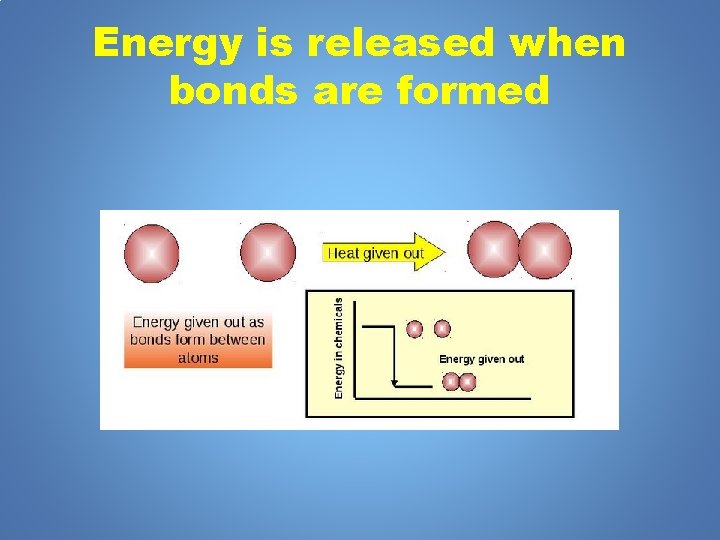
Energy is released when bonds are formed

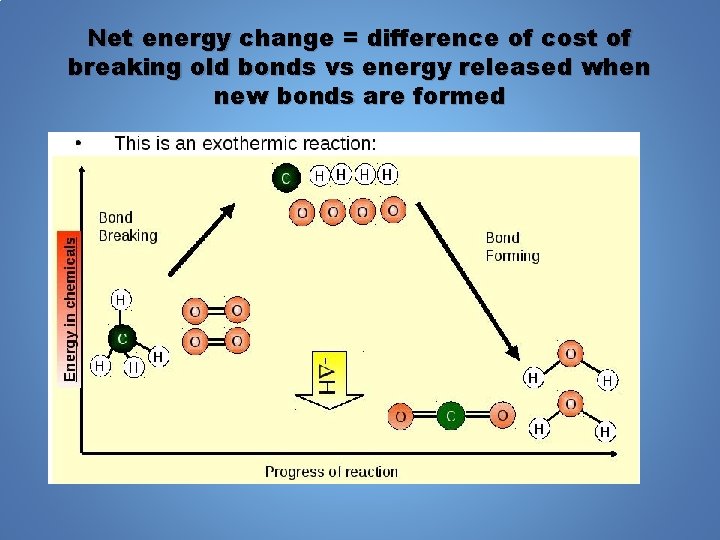
Net energy change = difference of cost of breaking old bonds vs energy released when new bonds are formed

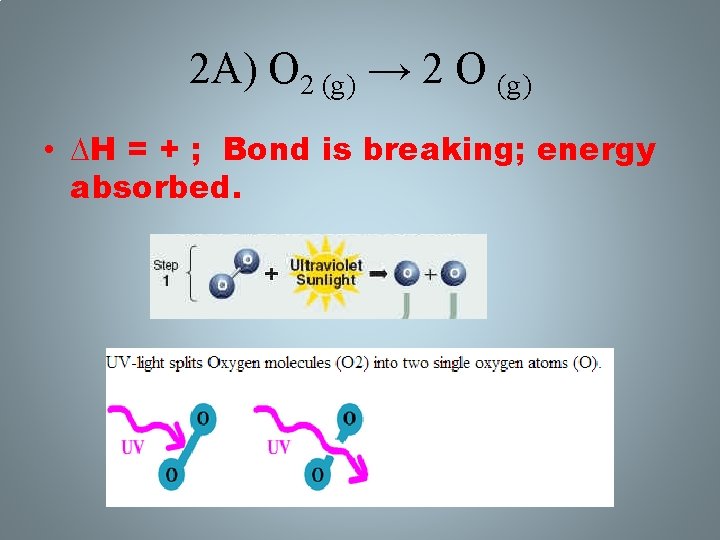
2 A) O 2 (g) → 2 O (g) • ∆H = + ; Bond is breaking; energy absorbed.

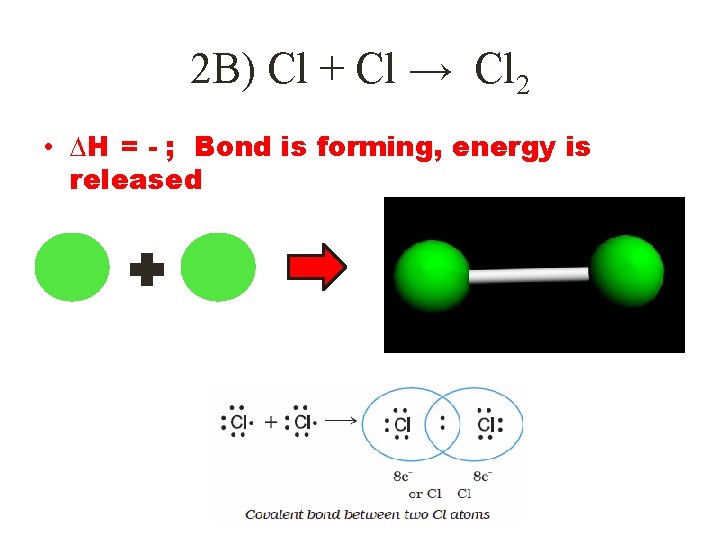
2 B) Cl + Cl → Cl 2 • ∆H = - ; Bond is forming, energy is released

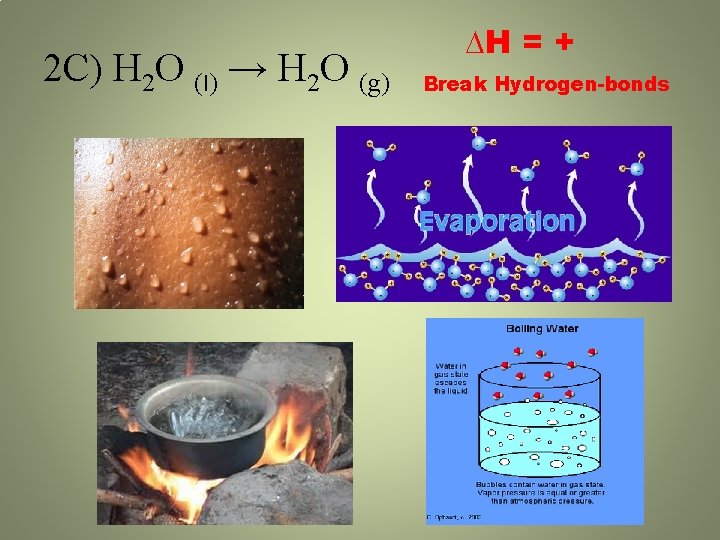
2 C) H 2 O (l) → H 2 O (g) ∆H = + Break Hydrogen-bonds


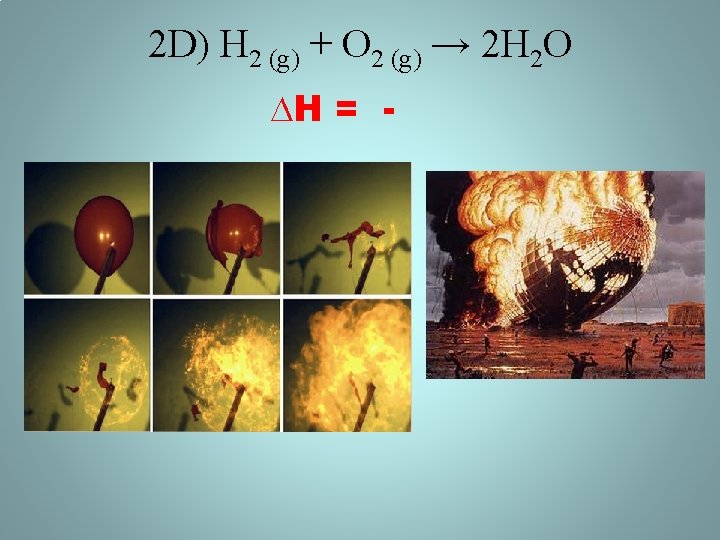
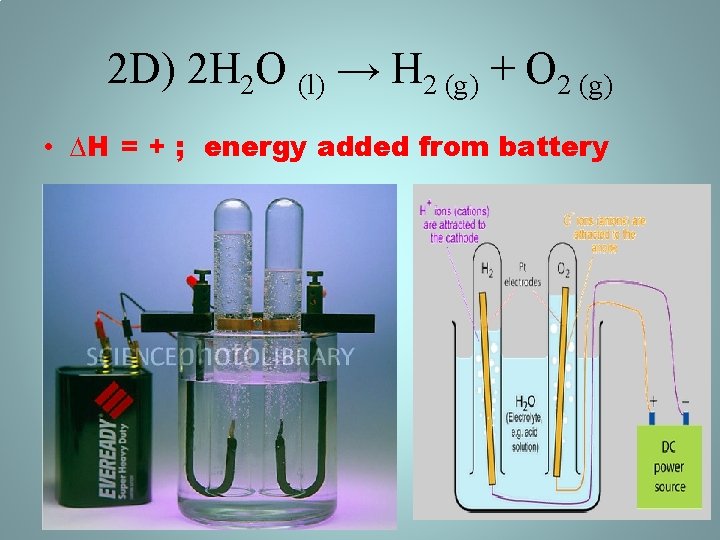
2 D) 2 H 2 O (l) → H 2 (g) + O 2 (g) • ∆H = + ; energy added from battery