Enthalpy EQ How do you predict the sign






- Slides: 17

Enthalpy EQ: How do you predict the sign of delta H?

A. Enthalpy (ΔH) 1. Enthalpy is the amount of heat in a system 2. In order to determine the amount of heat change in a reaction we use… ΔHrxn = Hproducts - Hreactants 3. Reactions can be…

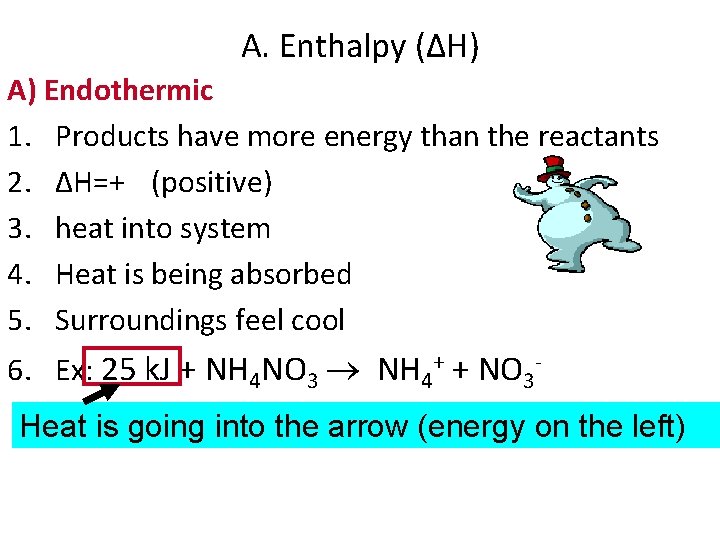
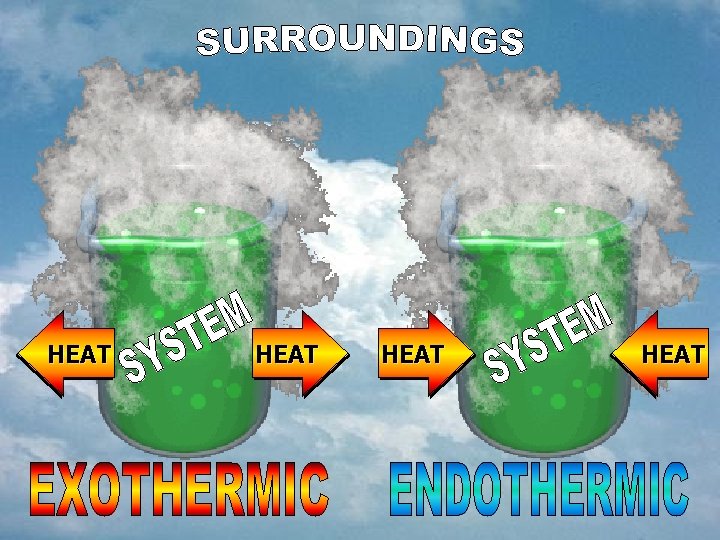
A. Enthalpy (ΔH) A) Endothermic 1. Products have more energy than the reactants 2. ΔH=+ (positive) 3. heat into system 4. Heat is being absorbed 5. Surroundings feel cool 6. Ex: 25 k. J + NH 4 NO 3 NH 4+ + NO 3 Heat is going into the arrow (energy on the left)

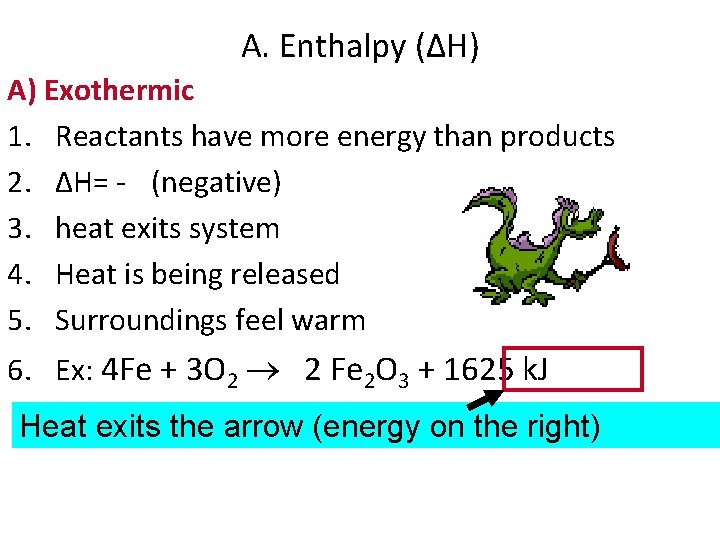
A. Enthalpy (ΔH) A) Exothermic 1. Reactants have more energy than products 2. ΔH= - (negative) 3. heat exits system 4. Heat is being released 5. Surroundings feel warm 6. Ex: 4 Fe + 3 O 2 2 Fe 2 O 3 + 1625 k. J Heat exits the arrow (energy on the right)

HEAT

Endo or Exo?

Endo or Exo

Endo or Exo?

Endo or Exo?

B. Enthalpy of Combustion (ΔHcomb) • 1. change in enthalpy when 1 mol of a substance is completely burned

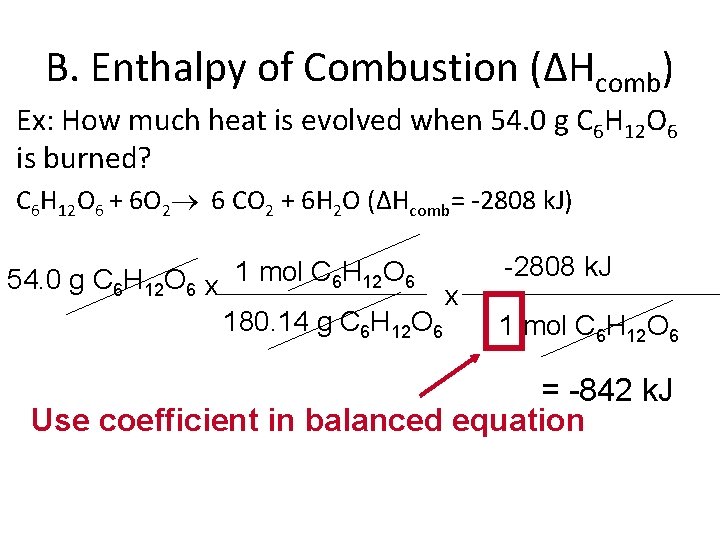
B. Enthalpy of Combustion (ΔHcomb) • Ex: How much heat is evolved when 54. 0 g C 6 H 12 O 6 is burned? C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O (ΔHcomb= -2808 k. J) 54. 0 g C 6 H 12 O 6 x 1 mol C 6 H 12 O 6 180. 14 g C 6 H 12 O 6 x -2808 k. J 1 mol C 6 H 12 O 6 = -842 k. J Use coefficient in balanced equation

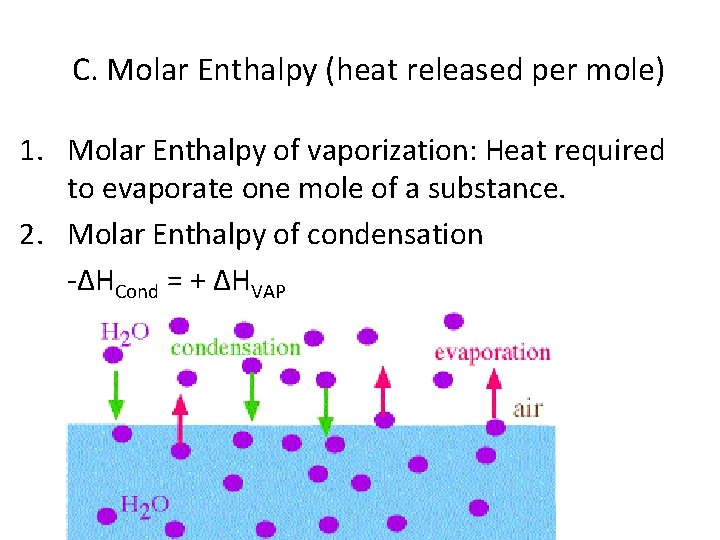
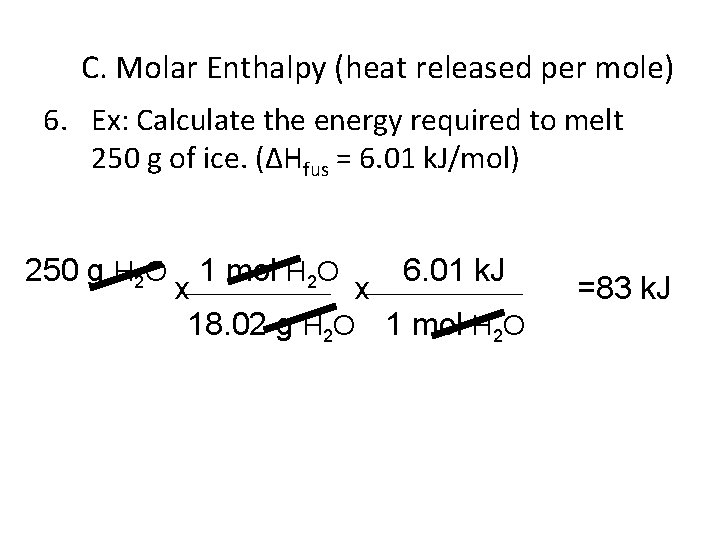
C. Molar Enthalpy (heat released per mole) 1. Molar Enthalpy of vaporization: Heat required to evaporate one mole of a substance. 2. Molar Enthalpy of condensation -ΔHCond = + ΔHVAP

C. Molar Enthalpy (heat released per mole) 3. Molar Enthalpy of fusion: Heat required to melt one mole of a substance 4. Molar enthalpy of freezing (ΔHsolid) solidification • -ΔHSolid = + ΔHFusion

C. Molar Enthalpy (heat released per mole) 5. If the ΔHfus is 6. 01 k. J/mol, than the ΔHsolid = -6. 01 k. J/mol

C. Molar Enthalpy (heat released per mole) 6. Ex: Calculate the energy required to melt 250 g of ice. (ΔHfus = 6. 01 k. J/mol) 250 g H 2 O 1 mol H 2 O 6. 01 k. J x x 18. 02 g H 2 O 1 mol H 2 O =83 k. J

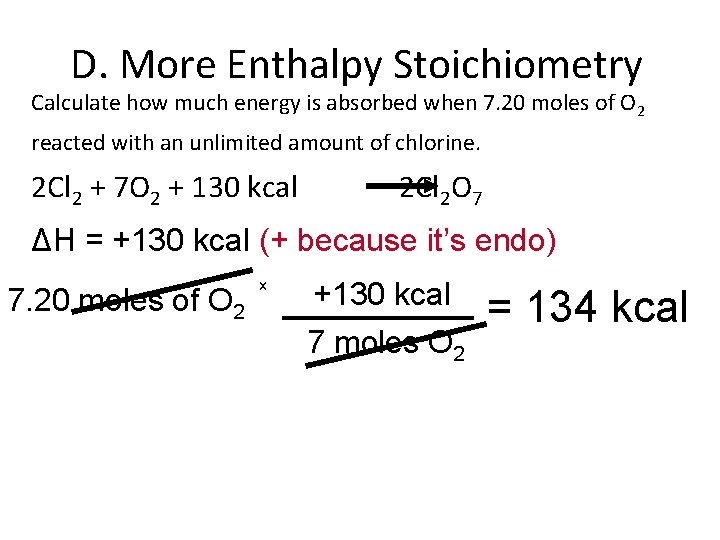
D. More Enthalpy Stoichiometry Calculate how much energy is absorbed when 7. 20 moles of O 2 reacted with an unlimited amount of chlorine. 2 Cl 2 + 7 O 2 + 130 kcal 2 Cl 2 O 7 ΔH = +130 kcal (+ because it’s endo) 7. 20 moles of O 2 x +130 kcal 7 moles O 2 = 134 kcal

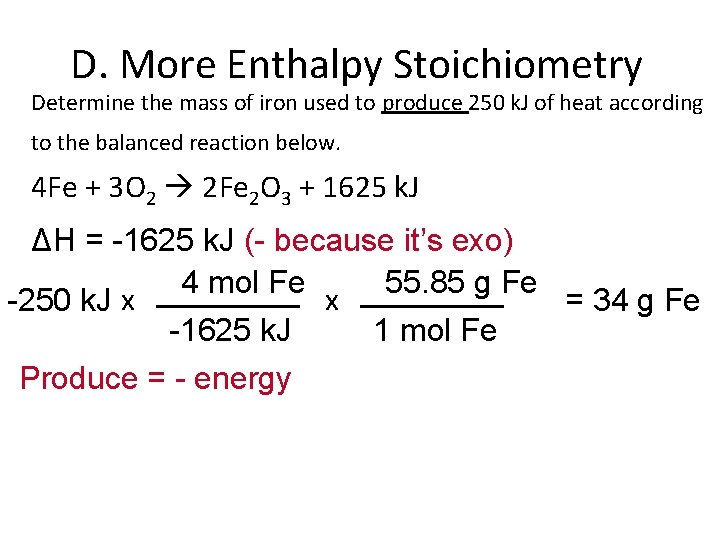
D. More Enthalpy Stoichiometry Determine the mass of iron used to produce 250 k. J of heat according to the balanced reaction below. 4 Fe + 3 O 2 2 Fe 2 O 3 + 1625 k. J ΔH = -1625 k. J (- because it’s exo) 4 mol Fe 55. 85 g Fe x -250 k. J x = 34 g Fe -1625 k. J 1 mol Fe Produce = - energy