Enthalpy Entropy and Gibbs Free Energy UNIT THEME

Enthalpy, Entropy, and Gibb’s Free Energy UNIT THEME: ENERGY

Collision Theory 1. The reactant particles must collide with each other. 2. The collisions must be of enough energy to overcome the activation energy 3. The reactants must form new bonds to produce products.

Defined as: Activation Energy The minimum energy required to start a chemical reaction. Everything has an activation energy
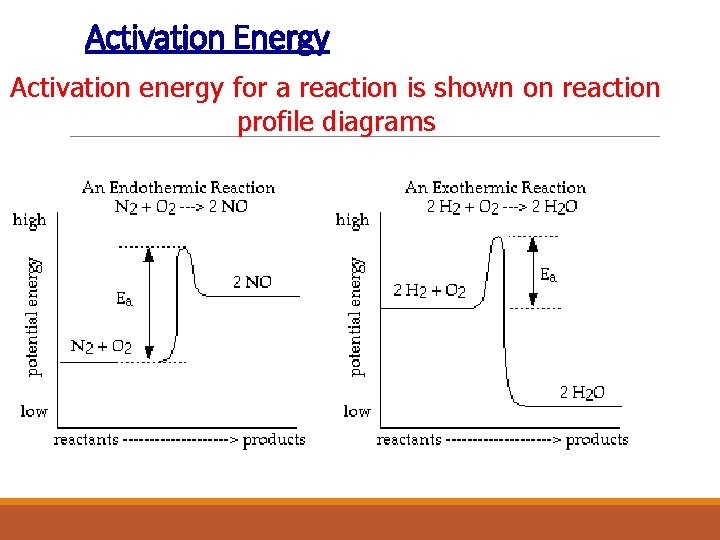
Activation Energy Activation energy for a reaction is shown on reaction profile diagrams

Changing the Rate of a Chemical Reaction To change the rate of a reaction one or more of the following things must happen: 1. Increase the number of collisions between the reactant particles 2. Increase the energy of the collisions. 3. Decrease the activation energy.
Effect of a Catalyst A catalyst is a substance that increases the speed of a reaction, without being used up. A catalyst can be ‘recovered at the end of a reaction and used again. A catalyst reduces the activation energy of a reaction.
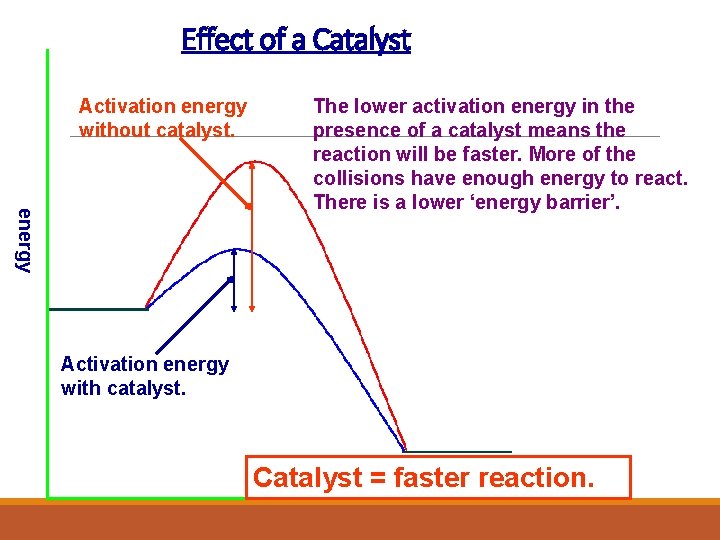
Effect of a Catalyst Activation energy without catalyst. energy The lower activation energy in the presence of a catalyst means the reaction will be faster. More of the collisions have enough energy to react. There is a lower ‘energy barrier’. Activation energy with catalyst. Catalyst = faster reaction.

Enthalpy of the reaction The total energy change associated with a chemical or physical change Given the symbol DHrxn = (energy of products) – (energy of reactants)
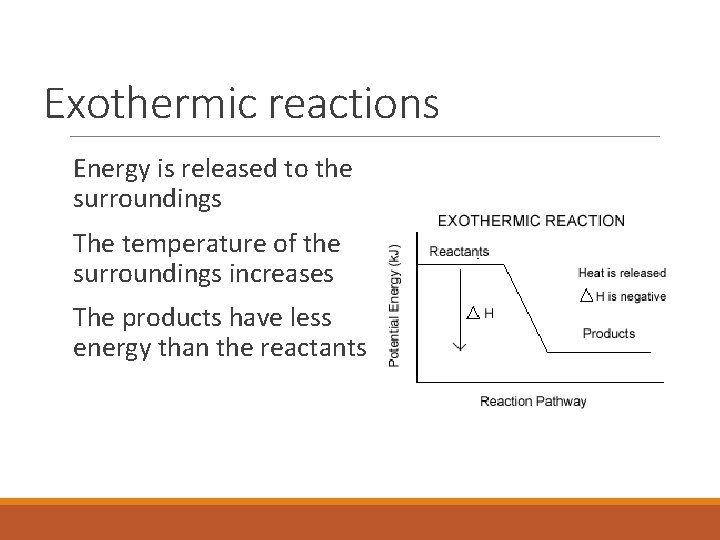
Exothermic reactions Energy is released to the surroundings The temperature of the surroundings increases The products have less energy than the reactants
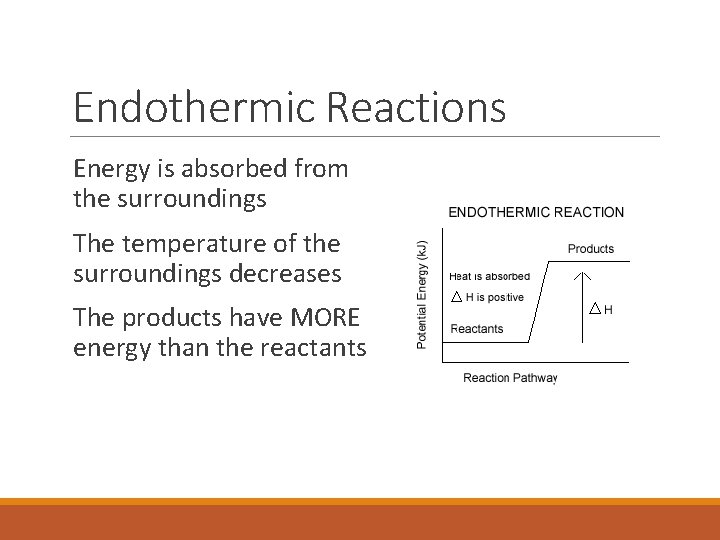
Endothermic Reactions Energy is absorbed from the surroundings The temperature of the surroundings decreases The products have MORE energy than the reactants
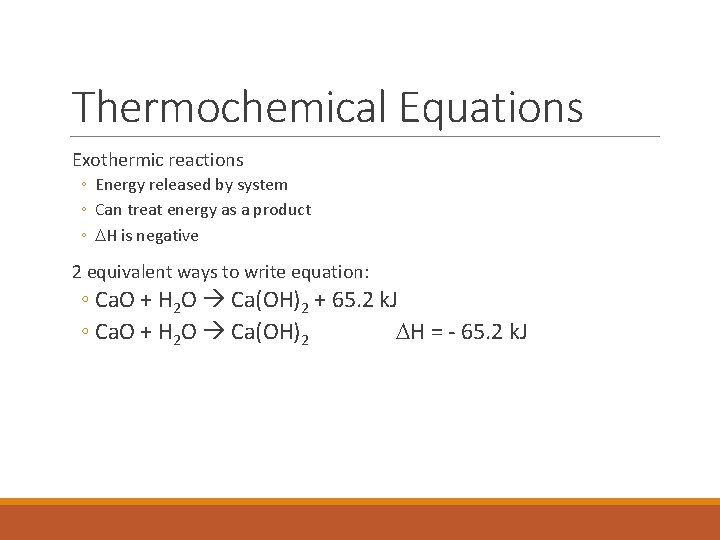
Thermochemical Equations Exothermic reactions ◦ Energy released by system ◦ Can treat energy as a product ◦ DH is negative 2 equivalent ways to write equation: ◦ Ca. O + H 2 O Ca(OH)2 + 65. 2 k. J ◦ Ca. O + H 2 O Ca(OH)2 DH = - 65. 2 k. J
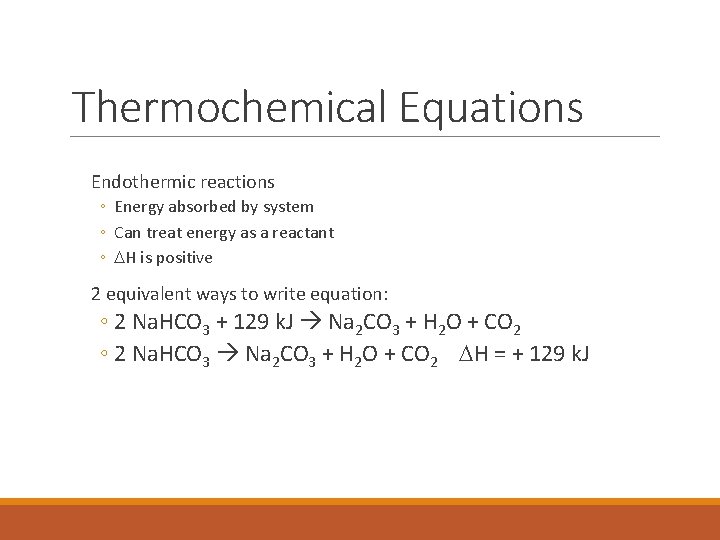
Thermochemical Equations Endothermic reactions ◦ Energy absorbed by system ◦ Can treat energy as a reactant ◦ DH is positive 2 equivalent ways to write equation: ◦ 2 Na. HCO 3 + 129 k. J Na 2 CO 3 + H 2 O + CO 2 ◦ 2 Na. HCO 3 Na 2 CO 3 + H 2 O + CO 2 DH = + 129 k. J

2 ways to manipulate thermochemical equations 1) Write equation backwards ◦ Sign of DH must change ◦ A+B C DH = + 123 k. J ◦ C A+B DH = - 123 k. J 2) Multiply everything by a coefficient ◦ Must multiply DH by coefficient, too! ◦ 3 A + 3 B 3 C DH = 3 (+123 k. J) = + 369 k. J

Problems with thermochemical equations Consider the equation: 2 Na. HCO 3 Na 2 CO 3 + H 2 O + CO 2 DH = + 129 k. J How many k. J would be required if 4. 5 moles Na. HCO 3 reacted?

Hess’ Law If you add two or more thermochemical equations to give a final equation, then add the heat changes to give the final enthalpy of reaction.
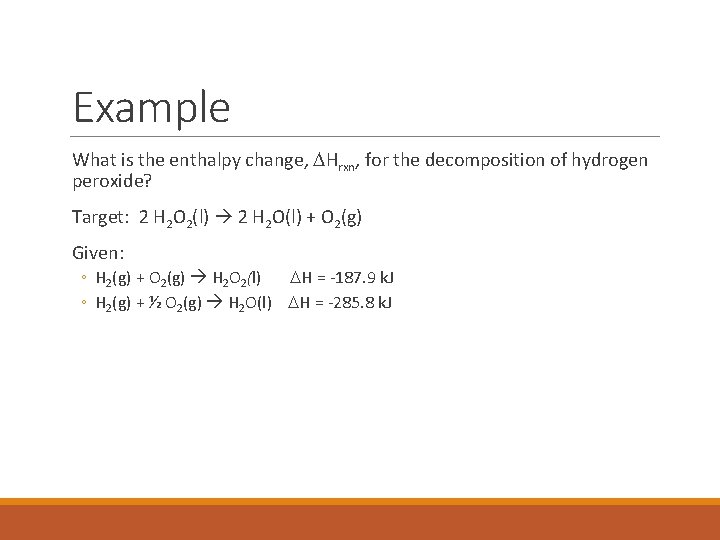
Example What is the enthalpy change, DHrxn, for the decomposition of hydrogen peroxide? Target: 2 H 2 O 2(l) 2 H 2 O(l) + O 2(g) Given: ◦ H 2(g) + O 2(g) H 2 O 2(l) DH = -187. 9 k. J ◦ H 2(g) + ½ O 2(g) H 2 O(l) DH = -285. 8 k. J
Standard Heats of Formation, o DH f The standard heat of formation of a compound is the change in enthalpy that accompanies the formation of one mole of a substance from its elements in their standard states. The heat of formation of elements in their standard states is arbitrarily set to zero.
Using heats of formation Thermodynamic stability: a measure of the energy required to decompose the compound ◦ Compounds with large, negative enthalpies of formation are thermodynamically stable ◦ Another way to do Hess’ Law! DHrxn = Sn. DHf(products) – Sm. DHf(reactants) ◦ where ◦ n represents the coefficients for the products ◦ m represents the coefficients for the reactants
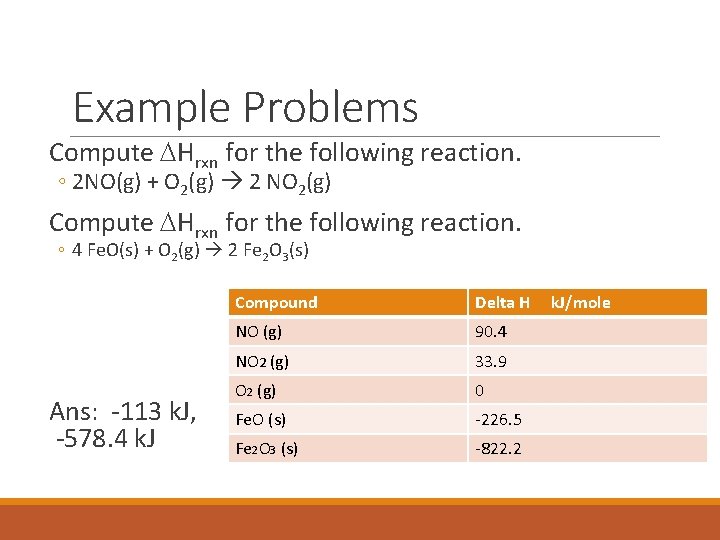
Example Problems Compute DHrxn for the following reaction. ◦ 2 NO(g) + O 2(g) 2 NO 2(g) Compute DHrxn for the following reaction. ◦ 4 Fe. O(s) + O 2(g) 2 Fe 2 O 3(s) Ans: -113 k. J, -578. 4 k. J Compound Delta H NO (g) 90. 4 NO 2 (g) 33. 9 O 2 (g) 0 Fe. O (s) -226. 5 Fe 2 O 3 (s) -822. 2 k. J/mole

Entropy Symbol: S A quantitative measure of the degree of disorder in a system ◦ The greater the disorder, the larger the value of S ◦ Solids have a high degree of order (low entropy) ◦ Gases have a low degree of order (high entropy) ◦ More particles (moles) results in higher entropy

Entropy, cont. Systems tend to proceed to higher disorder ◦ Examples ◦ Stirring sugar into your coffee ◦ The neatness of your room ◦ The order of cards in a pack of playing cards after shuffling
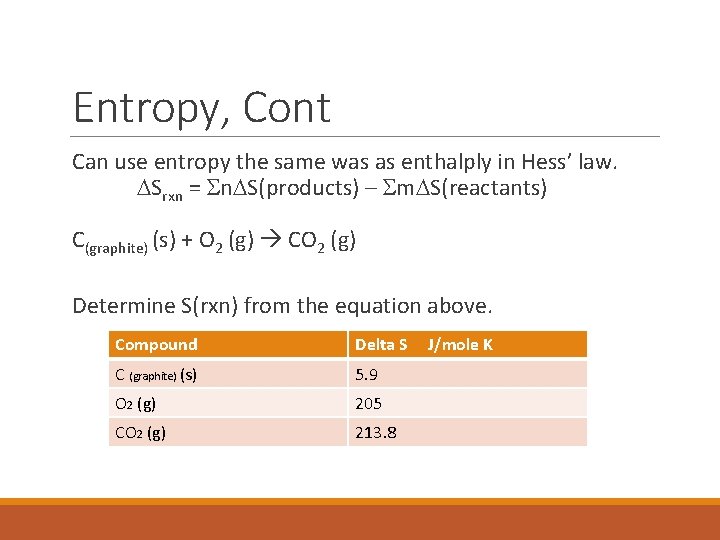
Entropy, Cont Can use entropy the same was as enthalply in Hess’ law. DSrxn = Sn. DS(products) – Sm. DS(reactants) C(graphite) (s) + O 2 (g) CO 2 (g) Determine S(rxn) from the equation above. Compound Delta S C (graphite) (s) 5. 9 O 2 (g) 205 CO 2 (g) 213. 8 J/mole K

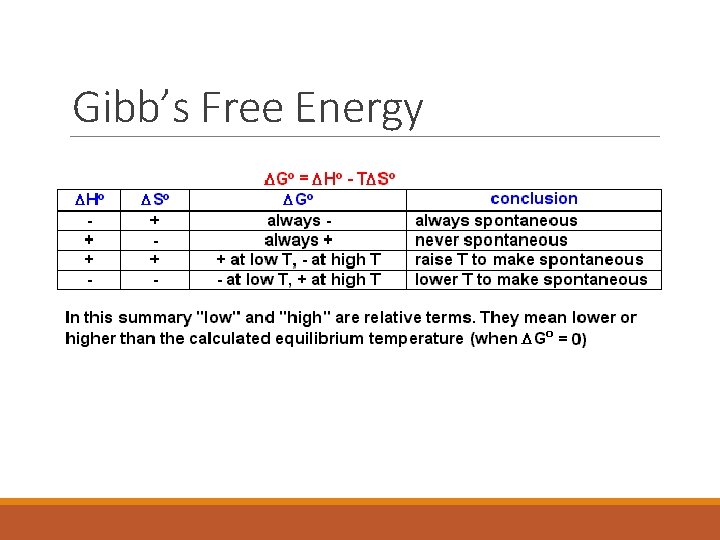
Will a reaction occur spontaneously? The answer depends on the balance between enthalpy and entropy Gibb’s Free Energy ◦ The energy available from the system to do useful work ◦ DG = DH – TDS ◦ Delta H is given in Kilojoules and Delta S is given in Joules so you MUST convert Delta S to kilojoules every time or you’ll get EVERY answer incorrect. ◦ T must be in Kelven. Add 273 to the degrees C you’re given.

Gibb’s Free Energy DG = DH – TDS If DG is negative, the reaction will occur spontaneously and can proceed on its own. If DG is positive, the reaction is nonspontaneous and needs a sustained energy input to proceed. If DG is zero, the reaction is at equilibrium (both the forward and reverse reactions take place!)
Gibb’s Free Energy
- Slides: 25