Enthalpy Entropy and Free Energy Enthalpy H heat














- Slides: 17

Enthalpy, Entropy, and Free Energy

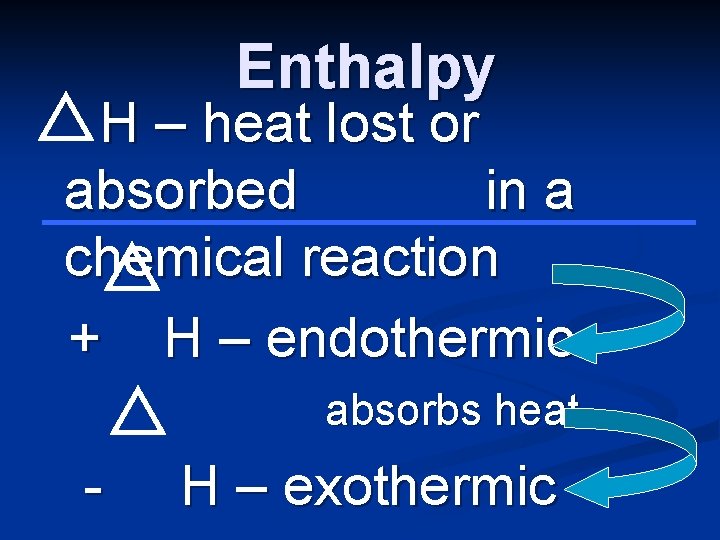
Enthalpy H – heat lost or absorbed in a chemical reaction + H – endothermic absorbs heat - H – exothermic


A measure of the disorder, or randomness, in a chemical reaction

Entropy and Phase Gases – most disorder Liquid – less disorder Solid – little disorder

S = Sfinal - Sinitial + S - S products more disordered than reactants products less disorder than reactants

What would be the sign of following: S for the 1. Liquid water becomes solid water Answer: Since the product is LESS DISORDERED, the sign of

2. Solid bromine sublimes into gaseous bromine Answer: Since the product is MORE DISORDER, the sign of S is positive

Gibbs Free Energy A measure that reflects the enthalpy, entropy, and temperature of a reaction that is used to determine spontaneity. - G = Spontaneous reaction + G = Nonspontaneous

G= H–T S The above equation is what is used to calculate the free energy change when given enthalpy, temperature, and entropy changes. For now, we will not do calculations, but you do need to know the equation!

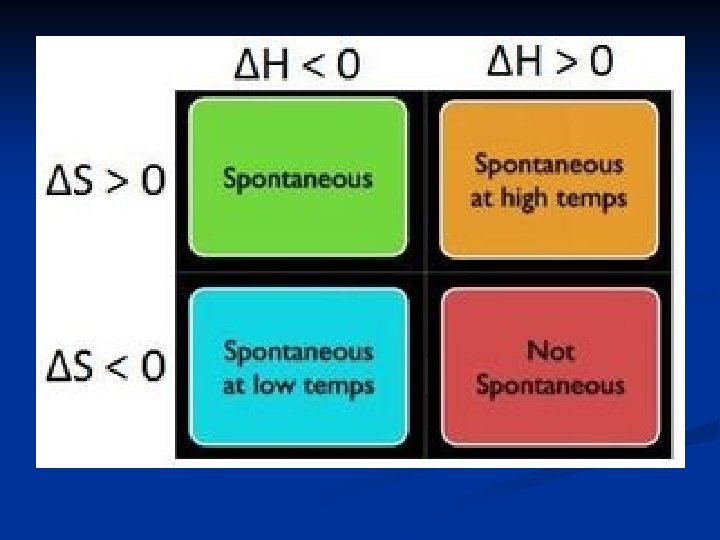
IMPORTANT All reactions would prefer to have LOW ENERGY (- H) HIGH DISORDER (+ S) Spontaneity ( G) depends on the above two factors.

Some scenarios: 1. A reaction results in low energy (- H) high disorder (+ S) It will always be SPONTANEOUS because it is getting everything it wants! So G will be negative!

2. A reaction results in high energy (+ H) low disorder (- S) It will always be NONSPONTANEOUS because it is getting nothing it wants! So G will be positive.

3. A reaction results in low energy (- H) low disorder (- S) One is good and one is bad so temperature is going to be important. In order for this reaction to be spontaneous, the temperature will have to be

4. A reaction results in high energy (+ H) high disorder (+ S) One is good and one is bad so temperature is going to be important. In order for this reaction to be spontaneous, the temperature will have to be

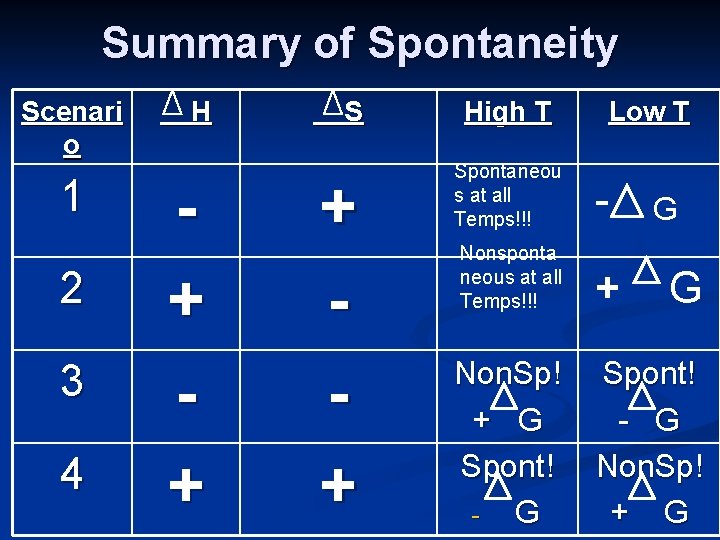
Summary of Spontaneity Scenari o 1 2 3 4 H + + S + + High T Low T Spontaneou s at all Temps!!! - Nonsponta neous at all Temps!!! + Non. Sp! + G Spont! G G G Spont! - G Non. Sp! + G
