Enthalpy Enthalpy For chemical reactions the change in


- Slides: 18

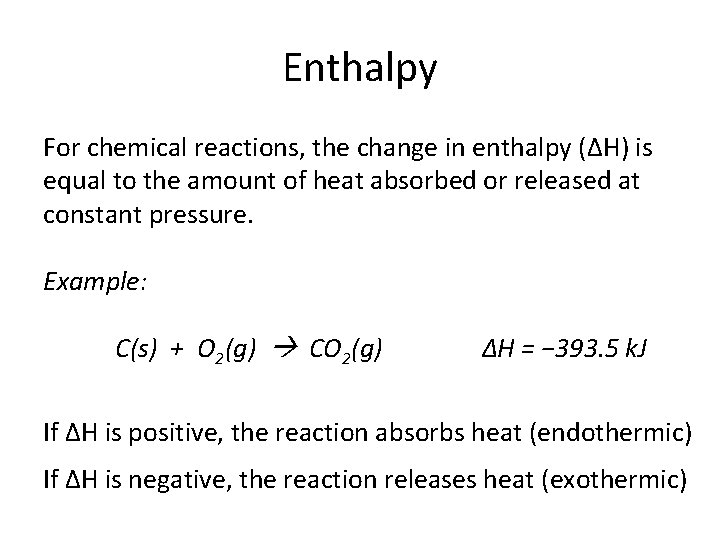
Enthalpy

Enthalpy For chemical reactions, the change in enthalpy (ΔH) is equal to the amount of heat absorbed or released at constant pressure. Example: C(s) + O 2(g) CO 2(g) ΔH = − 393. 5 k. J If ΔH is positive, the reaction absorbs heat (endothermic) If ΔH is negative, the reaction releases heat (exothermic)

Enthalpy For certain reactions, measuring this change in enthalpy may be difficult (especially if the reaction proceeds too slowly, or is part of a series of reactions). Hess’s Law states that the enthalpy change for a reaction is equal to the sum of the enthalpy changes for the individual steps.

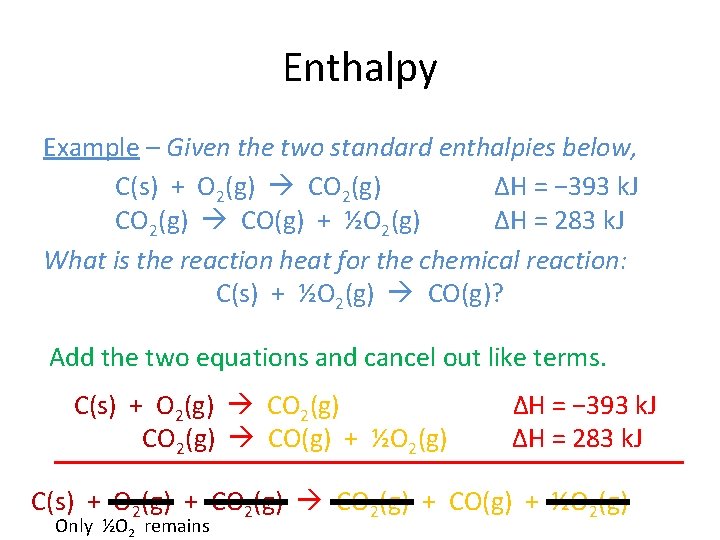
Enthalpy Example – Given the two standard enthalpies below, C(s) + O 2(g) CO 2(g) ΔH = − 393 k. J CO 2(g) CO(g) + ½O 2(g) ΔH = 283 k. J What is the reaction heat for the chemical reaction: C(s) + ½O 2(g) CO(g)? Add the two equations and cancel out like terms. C(s) + O 2(g) CO(g) + ½O 2(g) ΔH = − 393 k. J ΔH = 283 k. J C(s) + O 2(g) + CO(g) + ½O 2(g) Only ½O 2 remains

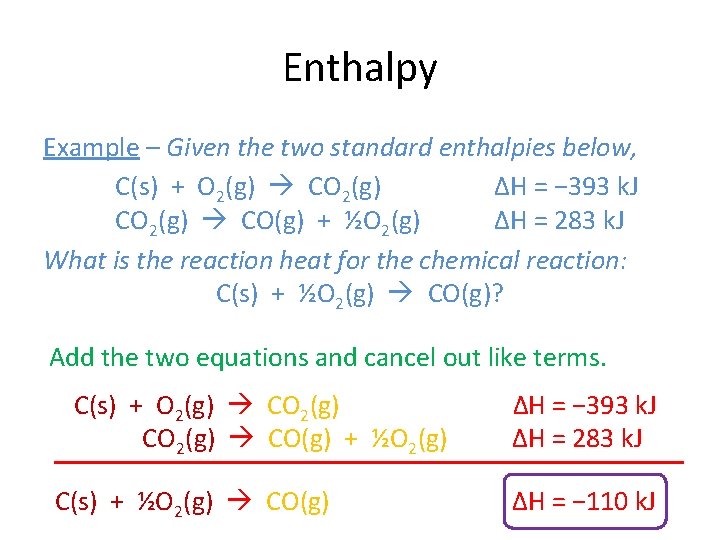
Enthalpy Example – Given the two standard enthalpies below, C(s) + O 2(g) CO 2(g) ΔH = − 393 k. J CO 2(g) CO(g) + ½O 2(g) ΔH = 283 k. J What is the reaction heat for the chemical reaction: C(s) + ½O 2(g) CO(g)? Add the two equations and cancel out like terms. C(s) + O 2(g) CO(g) + ½O 2(g) C(s) + ½O 2(g) CO(g) ΔH = − 393 k. J ΔH = 283 k. J ΔH = − 110 k. J

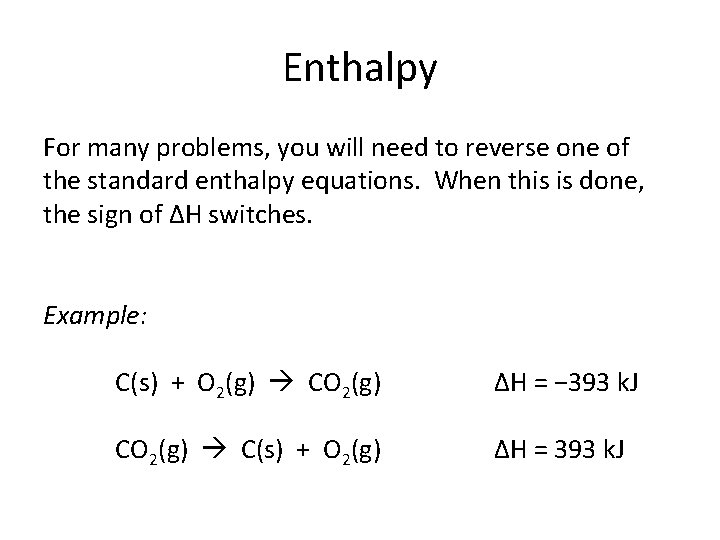
Enthalpy For many problems, you will need to reverse one of the standard enthalpy equations. When this is done, the sign of ΔH switches. Example: C(s) + O 2(g) CO 2(g) ΔH = − 393 k. J CO 2(g) C(s) + O 2(g) ΔH = 393 k. J

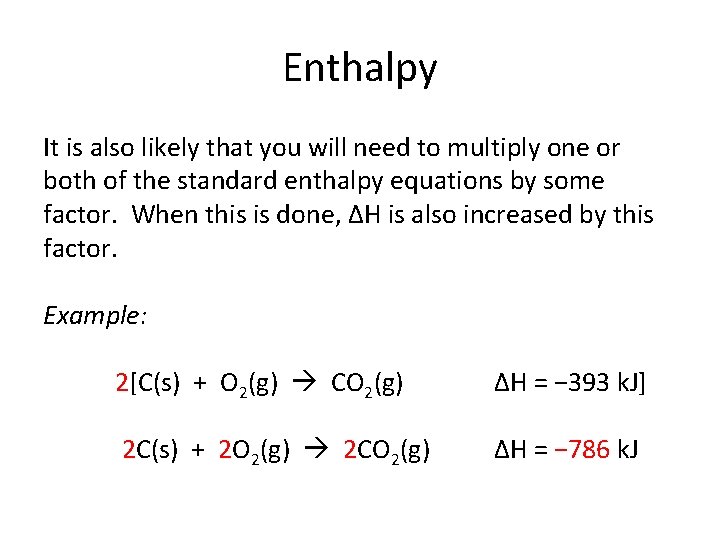
Enthalpy It is also likely that you will need to multiply one or both of the standard enthalpy equations by some factor. When this is done, ΔH is also increased by this factor. Example: 2[C(s) + O 2(g) CO 2(g) ΔH = − 393 k. J] 2 C(s) + 2 O 2(g) 2 CO 2(g) ΔH = − 786 k. J

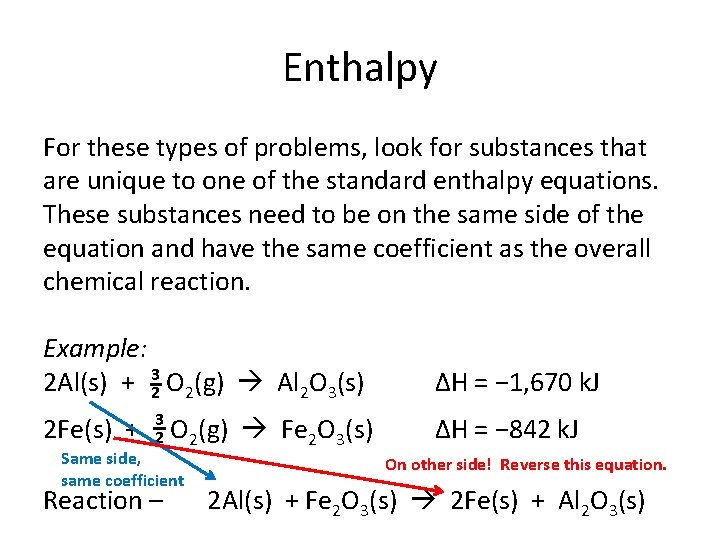
Enthalpy For these types of problems, look for substances that are unique to one of the standard enthalpy equations. These substances need to be on the same side of the equation and have the same coefficient as the overall chemical reaction. Example: 2 Al(s) + 32 O 2(g) Al 2 O 3(s) 2 Fe(s) + 3 2 O 2(g) Fe 2 O 3(s) Same side, same coefficient Reaction – ΔH = − 1, 670 k. J ΔH = − 842 k. J On other side! Reverse this equation. 2 Al(s) + Fe 2 O 3(s) 2 Fe(s) + Al 2 O 3(s)

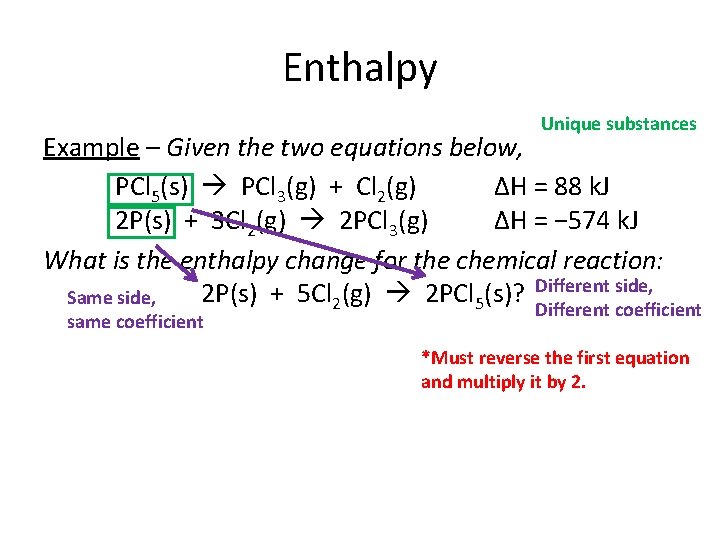
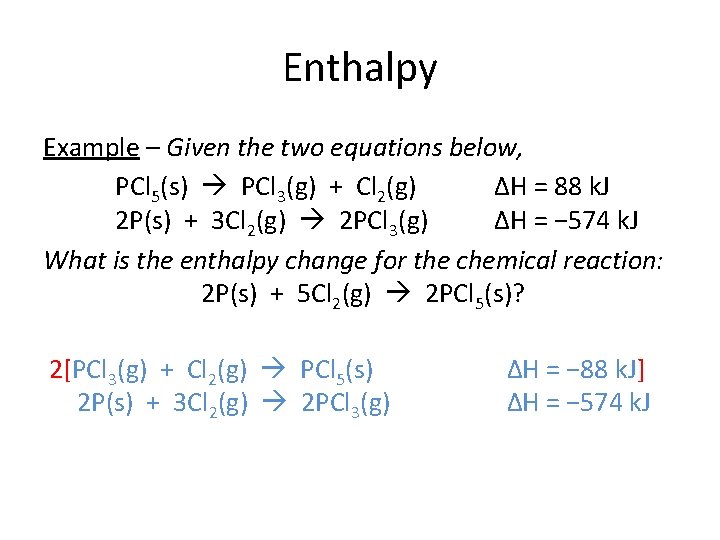
Enthalpy Unique substances Example – Given the two equations below, PCl 5(s) PCl 3(g) + Cl 2(g) ΔH = 88 k. J 2 P(s) + 3 Cl 2(g) 2 PCl 3(g) ΔH = − 574 k. J What is the enthalpy change for the chemical reaction: 2 P(s) + 5 Cl 2(g) 2 PCl 5(s)? Different side, Same side, same coefficient Different coefficient *Must reverse the first equation and multiply it by 2.

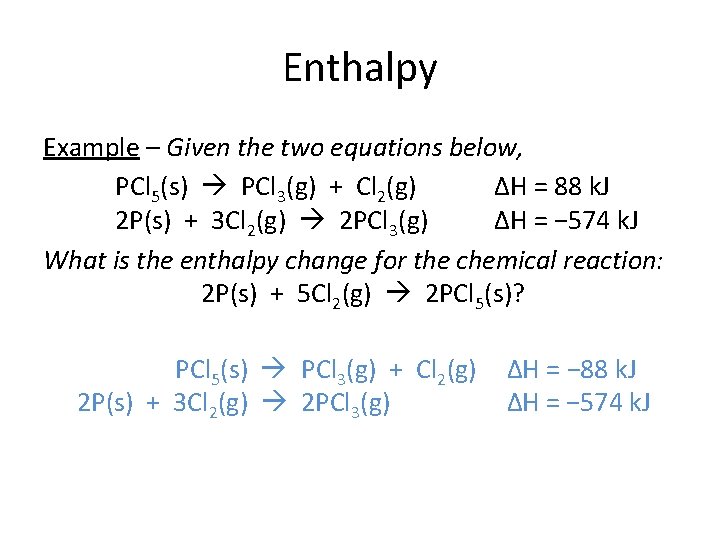
Enthalpy Example – Given the two equations below, PCl 5(s) PCl 3(g) + Cl 2(g) ΔH = 88 k. J 2 P(s) + 3 Cl 2(g) 2 PCl 3(g) ΔH = − 574 k. J What is the enthalpy change for the chemical reaction: 2 P(s) + 5 Cl 2(g) 2 PCl 5(s)? 2[PCl 3(g) + PCl Cl 25(g) (s) PCl 53(s) (g) + Cl 2(g) 2 P(s) + 3 Cl 2(g) 2 PCl 3(g) ΔH = − 88 − k. J] ΔH = − 574 k. J

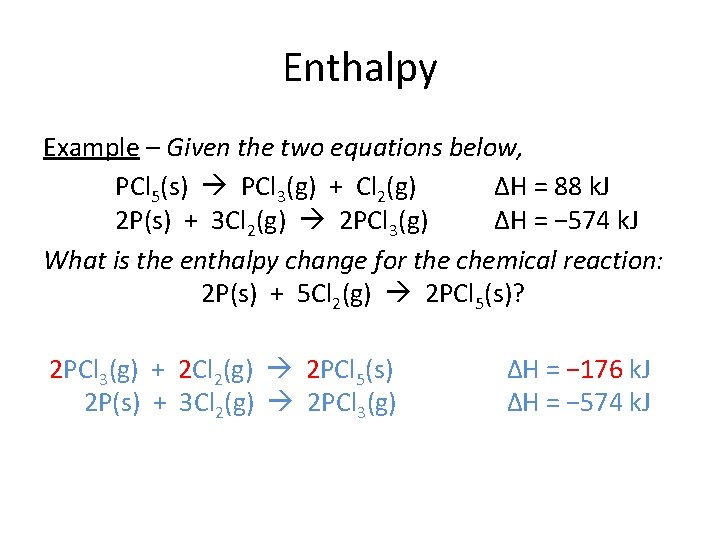
Enthalpy Example – Given the two equations below, PCl 5(s) PCl 3(g) + Cl 2(g) ΔH = 88 k. J 2 P(s) + 3 Cl 2(g) 2 PCl 3(g) ΔH = − 574 k. J What is the enthalpy change for the chemical reaction: 2 P(s) + 5 Cl 2(g) 2 PCl 5(s)? 2[PCl 3(g) + Cl 2(g) PCl 5(s) 2 P(s) + 3 Cl 2(g) 2 PCl 3(g) ΔH = − 88 k. J] ΔH = − 574 k. J

Enthalpy Example – Given the two equations below, PCl 5(s) PCl 3(g) + Cl 2(g) ΔH = 88 k. J 2 P(s) + 3 Cl 2(g) 2 PCl 3(g) ΔH = − 574 k. J What is the enthalpy change for the chemical reaction: 2 P(s) + 5 Cl 2(g) 2 PCl 5(s)? 2 PCl 3(g) + 2 Cl 2(g) 2 PCl 5(s) 2 P(s) + 3 Cl 2(g) 2 PCl 3(g) ΔH = − 176 k. J ΔH = − 574 k. J

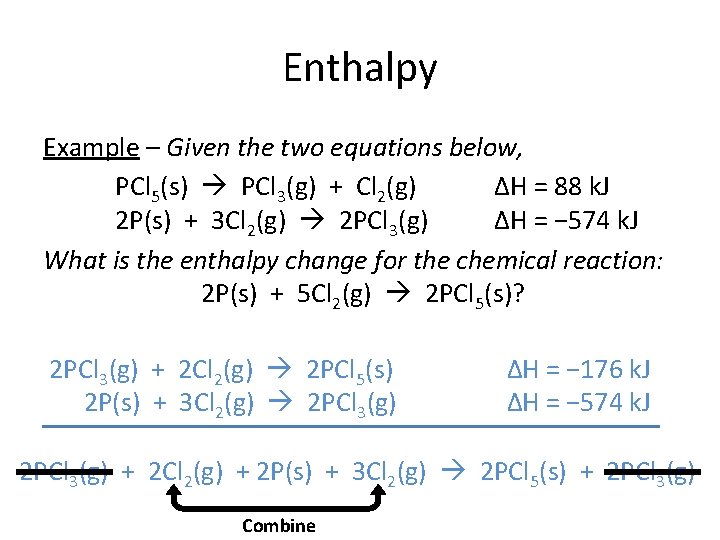
Enthalpy Example – Given the two equations below, PCl 5(s) PCl 3(g) + Cl 2(g) ΔH = 88 k. J 2 P(s) + 3 Cl 2(g) 2 PCl 3(g) ΔH = − 574 k. J What is the enthalpy change for the chemical reaction: 2 P(s) + 5 Cl 2(g) 2 PCl 5(s)? 2 PCl 3(g) + 2 Cl 2(g) 2 PCl 5(s) 2 P(s) + 3 Cl 2(g) 2 PCl 3(g) ΔH = − 176 k. J ΔH = − 574 k. J 2 PCl 3(g) + 2 Cl 2(g) + 2 P(s) + 3 Cl 2(g) 2 PCl 5(s) + 2 PCl 3(g) Combine

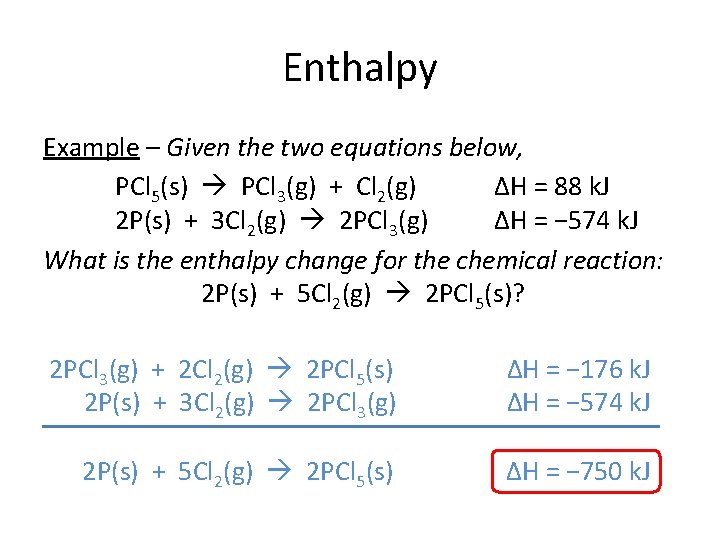
Enthalpy Example – Given the two equations below, PCl 5(s) PCl 3(g) + Cl 2(g) ΔH = 88 k. J 2 P(s) + 3 Cl 2(g) 2 PCl 3(g) ΔH = − 574 k. J What is the enthalpy change for the chemical reaction: 2 P(s) + 5 Cl 2(g) 2 PCl 5(s)? 2 PCl 3(g) + 2 Cl 2(g) 2 PCl 5(s) 2 P(s) + 3 Cl 2(g) 2 PCl 3(g) ΔH = − 176 k. J ΔH = − 574 k. J 2 P(s) + 5 Cl 2(g) 2 PCl 5(s) ΔH = − 750 k. J

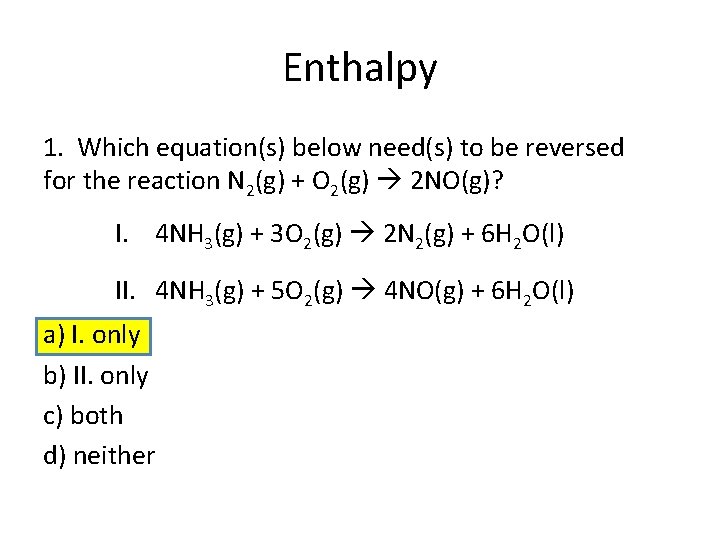
Enthalpy 1. Which equation(s) below need(s) to be reversed for the reaction N 2(g) + O 2(g) 2 NO(g)? I. 4 NH 3(g) + 3 O 2(g) 2 N 2(g) + 6 H 2 O(l) II. 4 NH 3(g) + 5 O 2(g) 4 NO(g) + 6 H 2 O(l) a) I. only b) II. only c) both d) neither

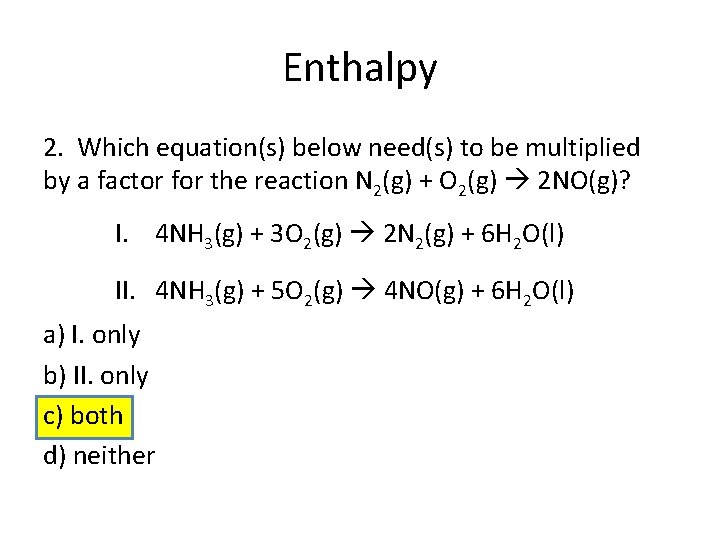
Enthalpy 2. Which equation(s) below need(s) to be multiplied by a factor for the reaction N 2(g) + O 2(g) 2 NO(g)? I. 4 NH 3(g) + 3 O 2(g) 2 N 2(g) + 6 H 2 O(l) II. 4 NH 3(g) + 5 O 2(g) 4 NO(g) + 6 H 2 O(l) a) I. only b) II. only c) both d) neither

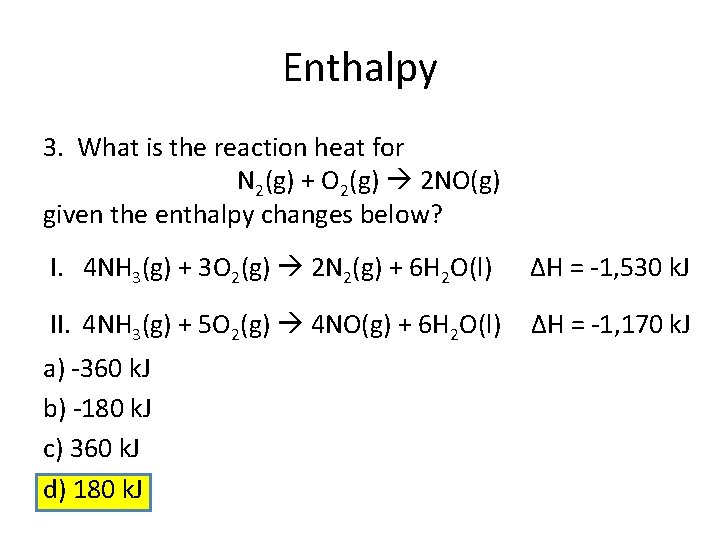
Enthalpy 3. What is the reaction heat for N 2(g) + O 2(g) 2 NO(g) given the enthalpy changes below? I. 4 NH 3(g) + 3 O 2(g) 2 N 2(g) + 6 H 2 O(l) ΔH = -1, 530 k. J II. 4 NH 3(g) + 5 O 2(g) 4 NO(g) + 6 H 2 O(l) ΔH = -1, 170 k. J a) -360 k. J b) -180 k. J c) 360 k. J d) 180 k. J

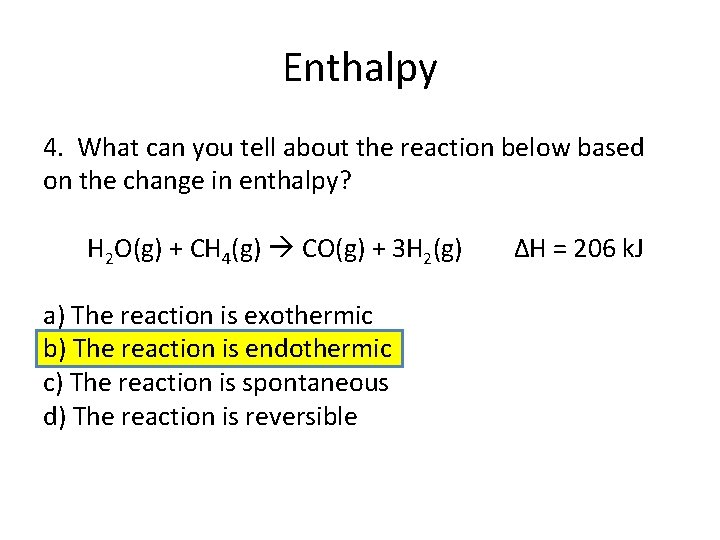
Enthalpy 4. What can you tell about the reaction below based on the change in enthalpy? H 2 O(g) + CH 4(g) CO(g) + 3 H 2(g) a) The reaction is exothermic b) The reaction is endothermic c) The reaction is spontaneous d) The reaction is reversible ΔH = 206 k. J