ENT 266 PRINCIPLES OF ENGINEERING MATERIALS SEMESTER 1








- Slides: 37

ENT 266 PRINCIPLES OF ENGINEERING MATERIALS SEMESTER 1 ACADEMIC SESSION 2017/2018 Chapter 09: Corrosion and Degradation of Materials DR HASIMAH ALI School of Mechatronic Engineering hasimahali@unimap. edu. my

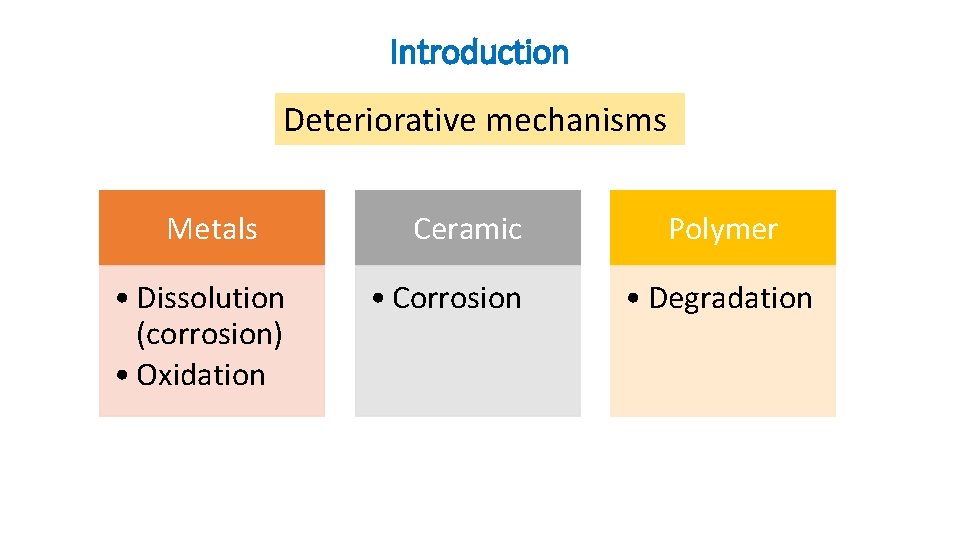
Introduction Deteriorative mechanisms Metals • Dissolution (corrosion) • Oxidation Ceramic • Corrosion Polymer • Degradation

Corrosion and Degradation ISSUES TO ADDRESS. . . • Why does corrosion occur? • What metals are most likely to corrode? • How do temperature and environment affect corrosion rate? • How do we suppress corrosion? Photos courtesy L. M. Maestas, Sandia National Labs. Used with permission.

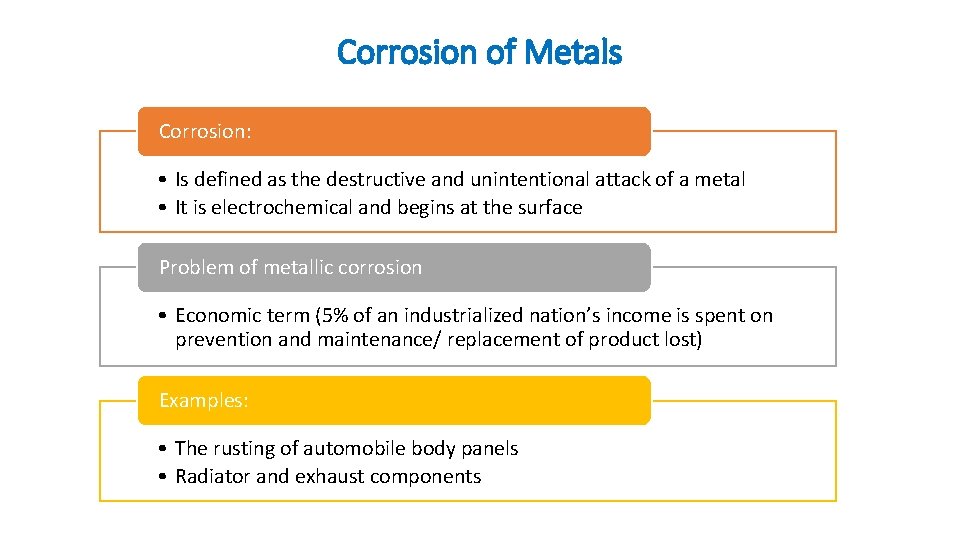
Corrosion of Metals Corrosion: • Is defined as the destructive and unintentional attack of a metal • It is electrochemical and begins at the surface Problem of metallic corrosion • Economic term (5% of an industrialized nation’s income is spent on prevention and maintenance/ replacement of product lost) Examples: • The rusting of automobile body panels • Radiator and exhaust components

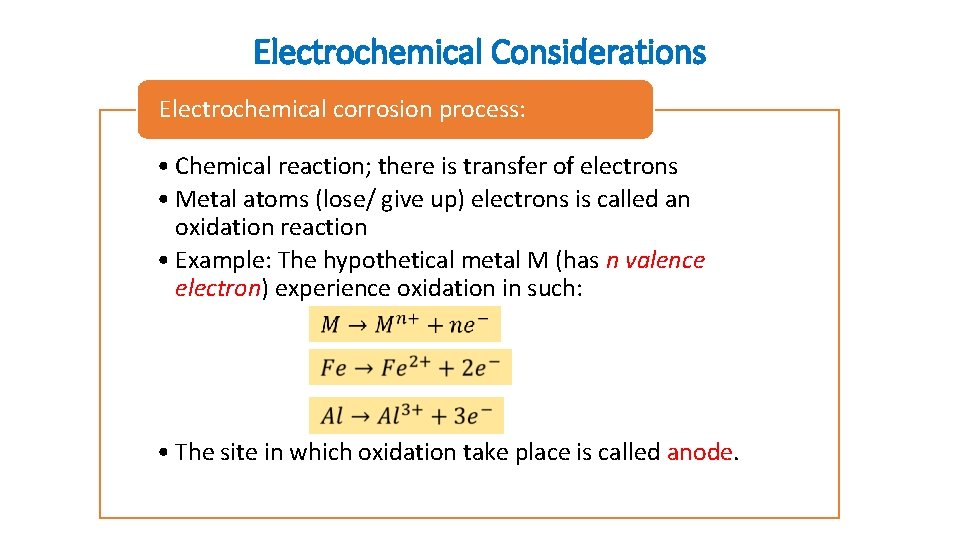
Electrochemical Considerations Electrochemical corrosion process: • Chemical reaction; there is transfer of electrons • Metal atoms (lose/ give up) electrons is called an oxidation reaction • Example: The hypothetical metal M (has n valence electron) experience oxidation in such: • The site in which oxidation take place is called anode.

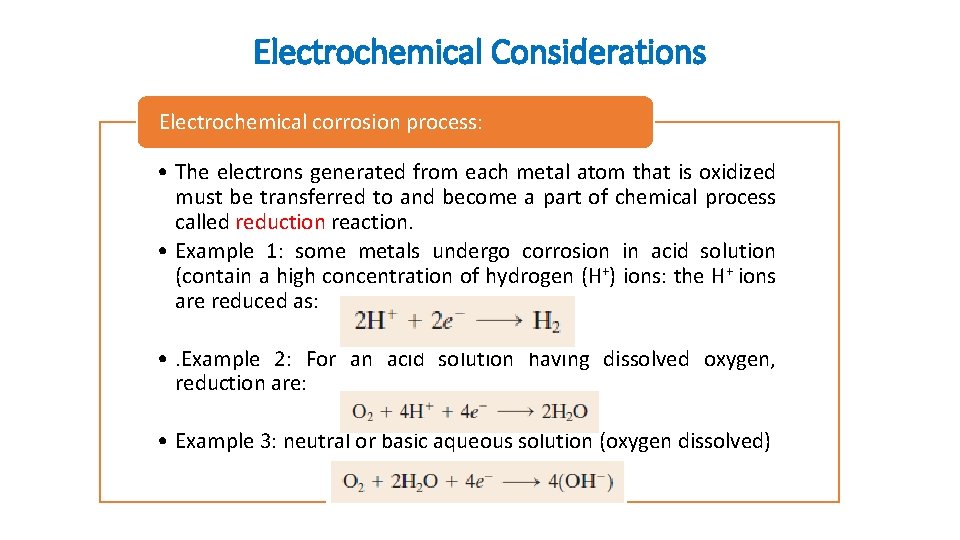
Electrochemical Considerations Electrochemical corrosion process: • The electrons generated from each metal atom that is oxidized must be transferred to and become a part of chemical process called reduction reaction. • Example 1: some metals undergo corrosion in acid solution (contain a high concentration of hydrogen (H+) ions: the H+ ions are reduced as: • . Example 2: For an acid solution having dissolved oxygen, reduction are: • Example 3: neutral or basic aqueous solution (oxygen dissolved)

Electrochemical corrosion process: Electrochemical Considerations • That location at which reduction occurs is called the cathode. • An overall electrochemical reaction must consist of at least one oxidation and one reduction reaction, and will be the sum of them; often the individual oxidation and reduction reactions are termed half-reactions. • The total rate of oxidation must equal the total rate of reduction, or all electrons generated through oxidation must be consumed by reduction. • Example 1: zinc metal immersed in an acid solution containing H+ ions. • Zinc will experience oxidation/ corrosion. These electrons may be transferred to an adjacent region at which H+ are reduced as:

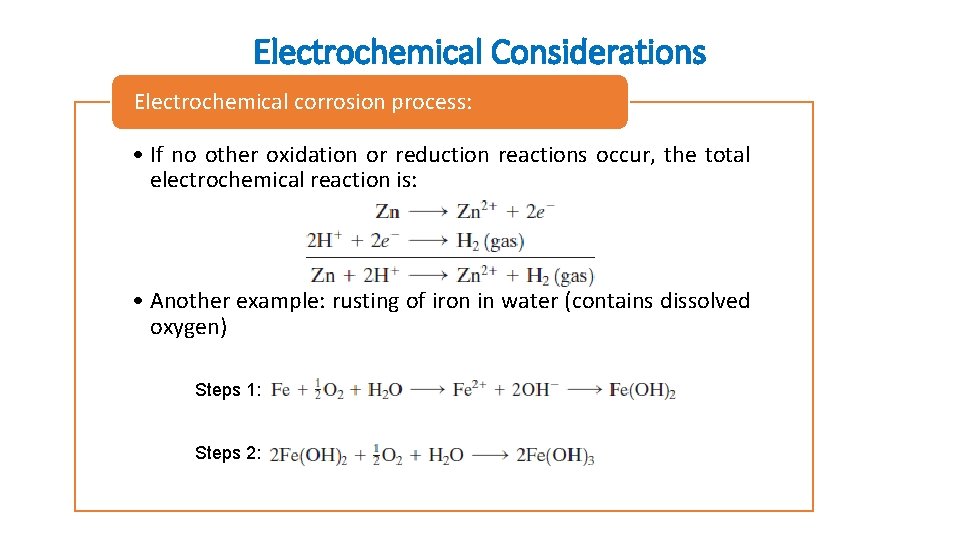
Electrochemical Considerations Electrochemical corrosion process: • If no other oxidation or reduction reactions occur, the total electrochemical reaction is: • Another example: rusting of iron in water (contains dissolved oxygen) Steps 1: Steps 2:

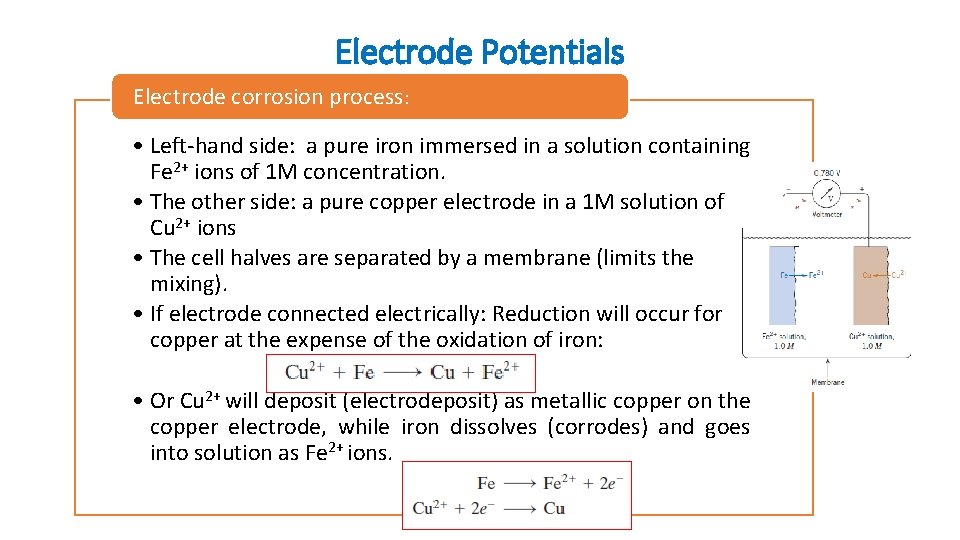
Electrode Potentials Electrode corrosion process: • Left-hand side: a pure iron immersed in a solution containing Fe 2+ ions of 1 M concentration. • The other side: a pure copper electrode in a 1 M solution of Cu 2+ ions • The cell halves are separated by a membrane (limits the mixing). • If electrode connected electrically: Reduction will occur for copper at the expense of the oxidation of iron: • Or Cu 2+ will deposit (electrodeposit) as metallic copper on the copper electrode, while iron dissolves (corrodes) and goes into solution as Fe 2+ ions.

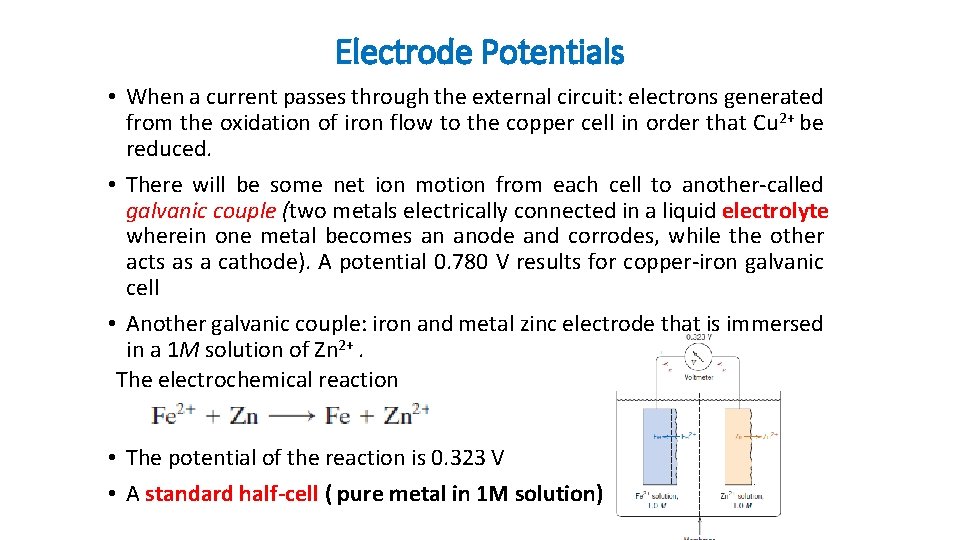
Electrode Potentials • When a current passes through the external circuit: electrons generated from the oxidation of iron flow to the copper cell in order that Cu 2+ be reduced. • There will be some net ion motion from each cell to another-called galvanic couple (two metals electrically connected in a liquid electrolyte wherein one metal becomes an anode and corrodes, while the other acts as a cathode). A potential 0. 780 V results for copper-iron galvanic cell • Another galvanic couple: iron and metal zinc electrode that is immersed in a 1 M solution of Zn 2+. The electrochemical reaction • The potential of the reaction is 0. 323 V • A standard half-cell ( pure metal in 1 M solution)

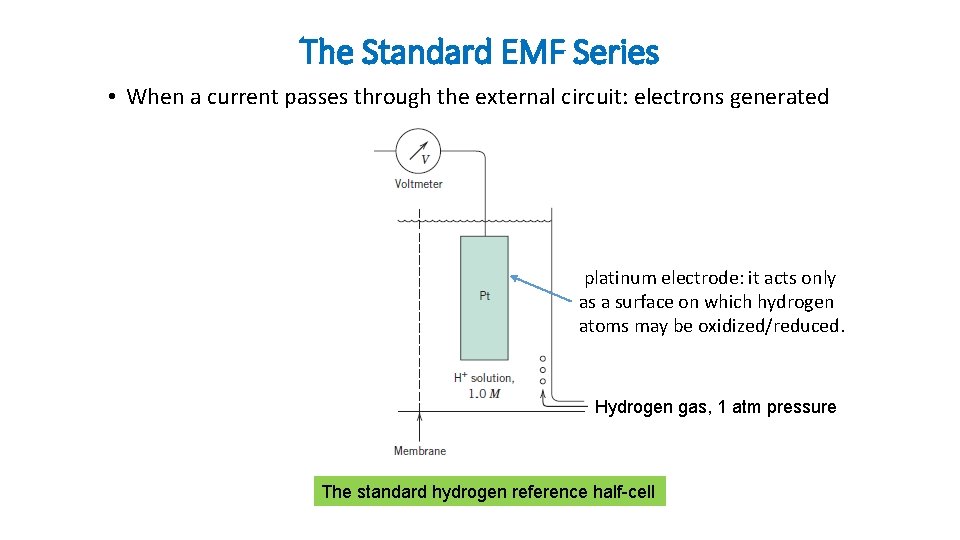
The Standard EMF Series • When a current passes through the external circuit: electrons generated platinum electrode: it acts only as a surface on which hydrogen atoms may be oxidized/reduced. Hydrogen gas, 1 atm pressure The standard hydrogen reference half-cell

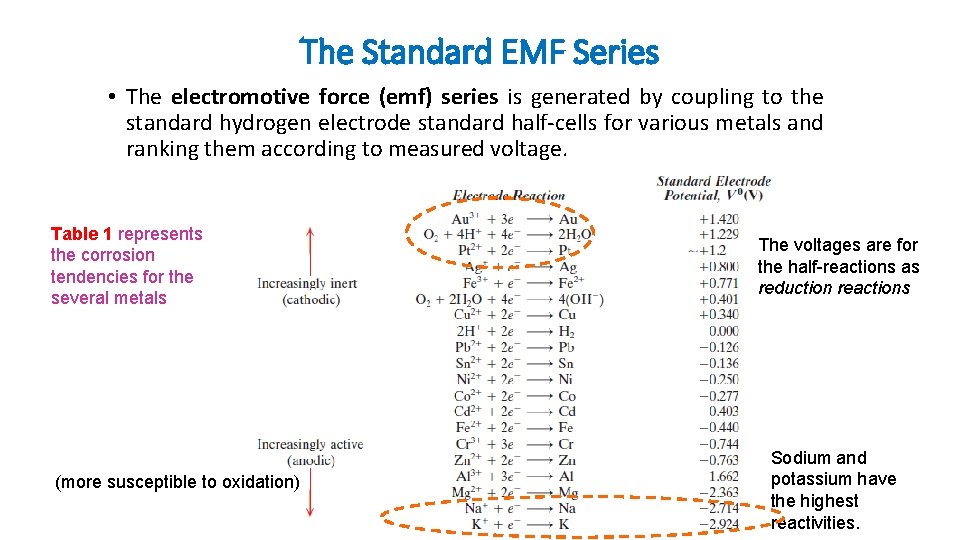
The Standard EMF Series • The electromotive force (emf) series is generated by coupling to the standard hydrogen electrode standard half-cells for various metals and ranking them according to measured voltage. Table 1 represents the corrosion tendencies for the several metals (more susceptible to oxidation) The voltages are for the half-reactions as reduction reactions Sodium and potassium have the highest reactivities.

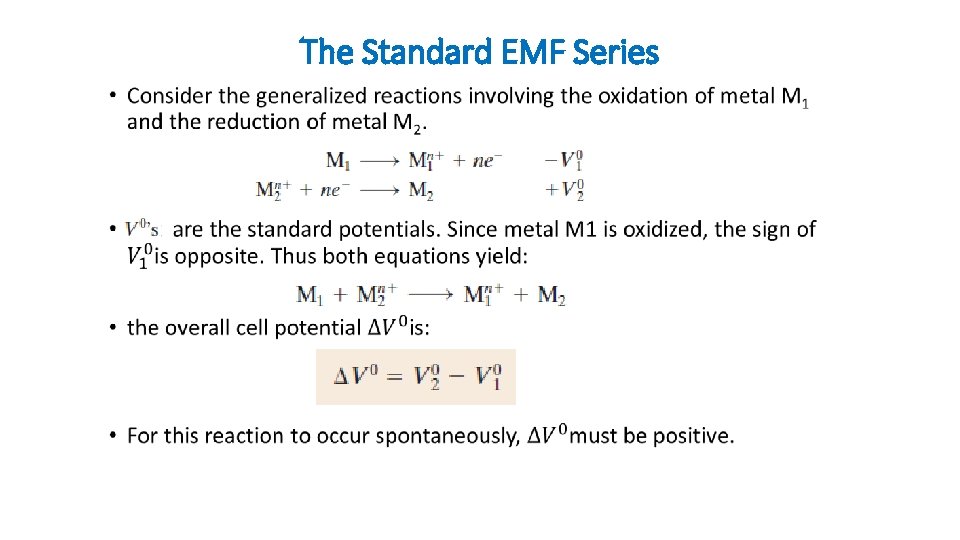
The Standard EMF Series •

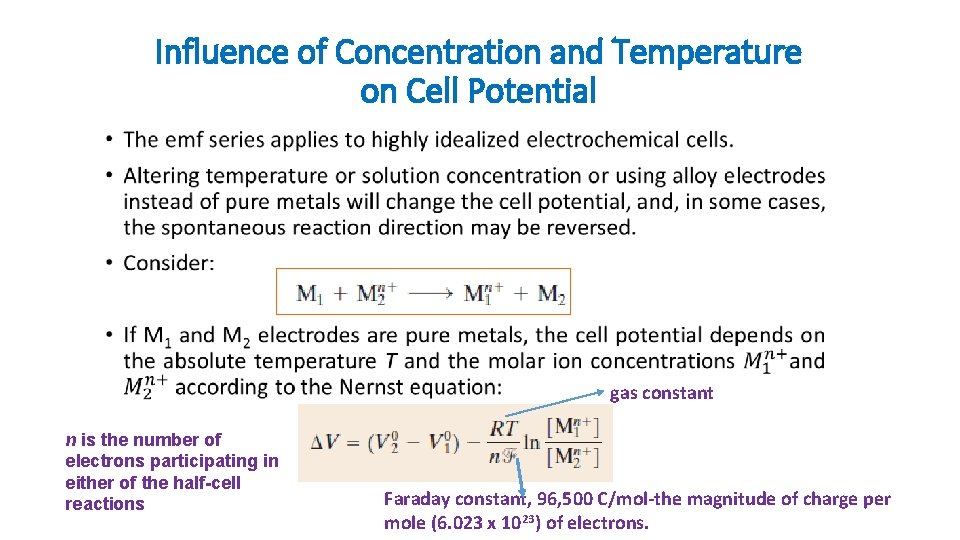
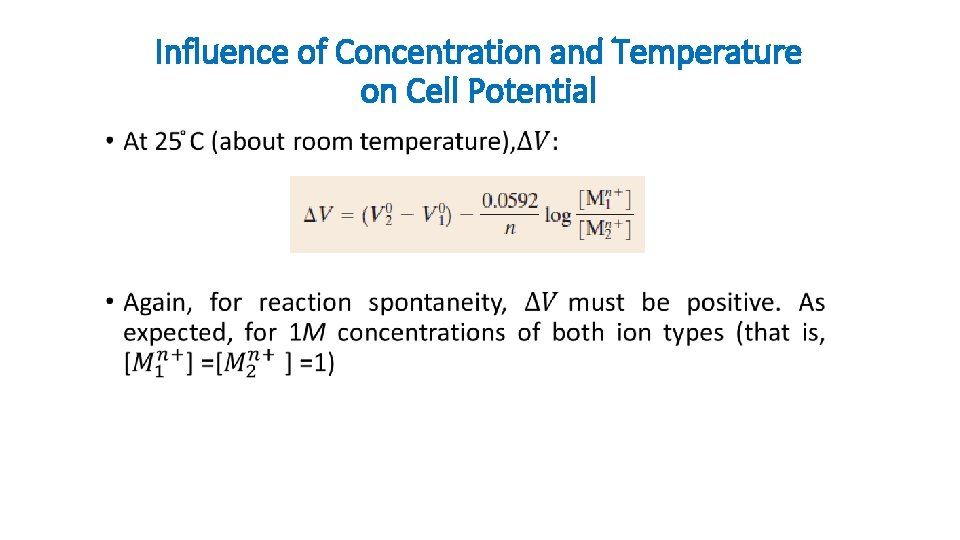
Influence of Concentration and Temperature on Cell Potential • gas constant n is the number of electrons participating in either of the half-cell reactions Faraday constant, 96, 500 C/mol-the magnitude of charge per mole (6. 023 x 1023) of electrons.

Influence of Concentration and Temperature on Cell Potential •

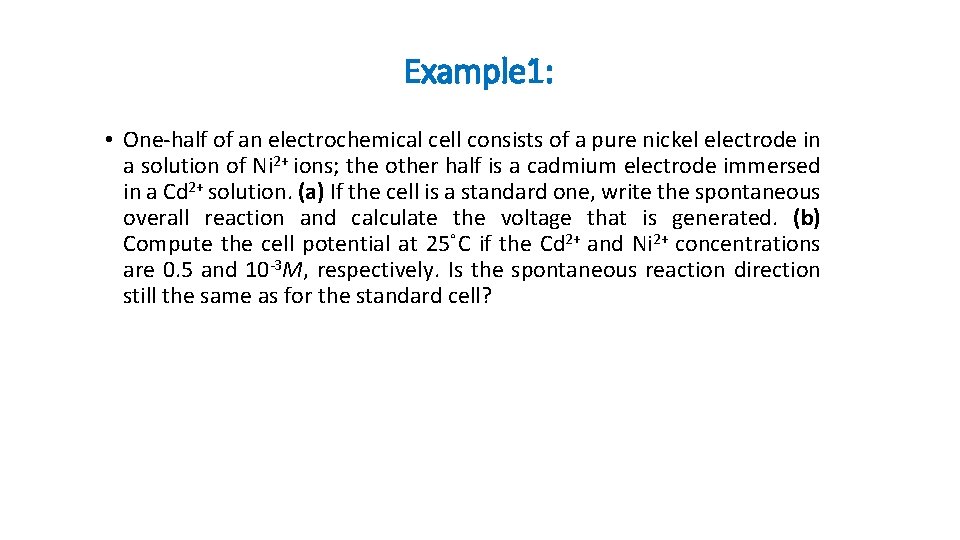
Example 1: • One-half of an electrochemical cell consists of a pure nickel electrode in a solution of Ni 2+ ions; the other half is a cadmium electrode immersed in a Cd 2+ solution. (a) If the cell is a standard one, write the spontaneous overall reaction and calculate the voltage that is generated. (b) Compute the cell potential at 25 C if the Cd 2+ and Ni 2+ concentrations are 0. 5 and 10 -3 M, respectively. Is the spontaneous reaction direction still the same as for the standard cell?

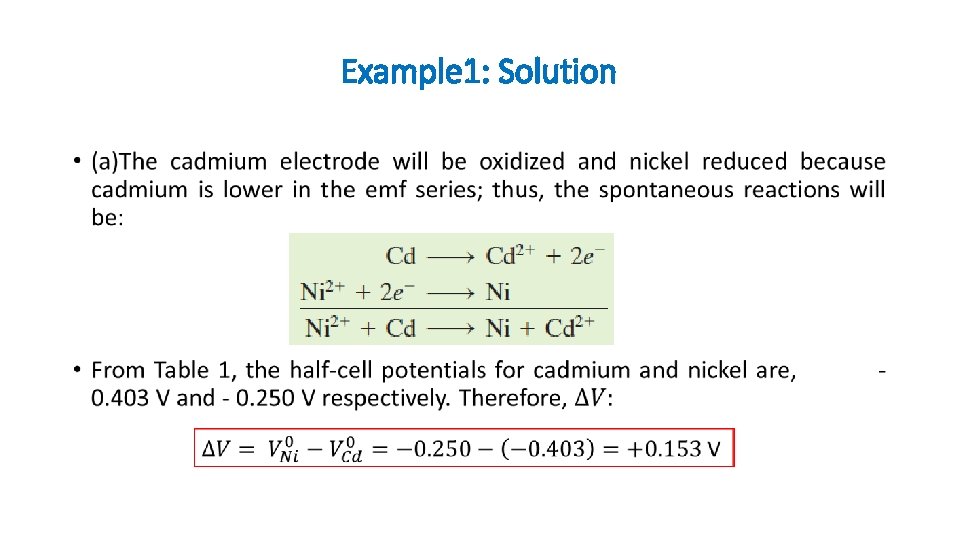
Example 1: Solution •

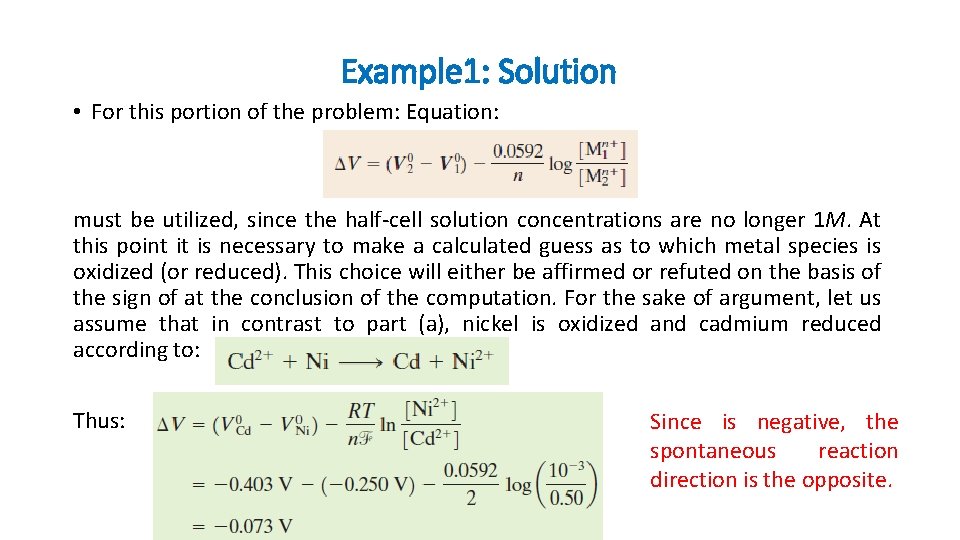
Example 1: Solution • For this portion of the problem: Equation: must be utilized, since the half-cell solution concentrations are no longer 1 M. At this point it is necessary to make a calculated guess as to which metal species is oxidized (or reduced). This choice will either be affirmed or refuted on the basis of the sign of at the conclusion of the computation. For the sake of argument, let us assume that in contrast to part (a), nickel is oxidized and cadmium reduced according to: Thus: Since is negative, the spontaneous reaction direction is the opposite.

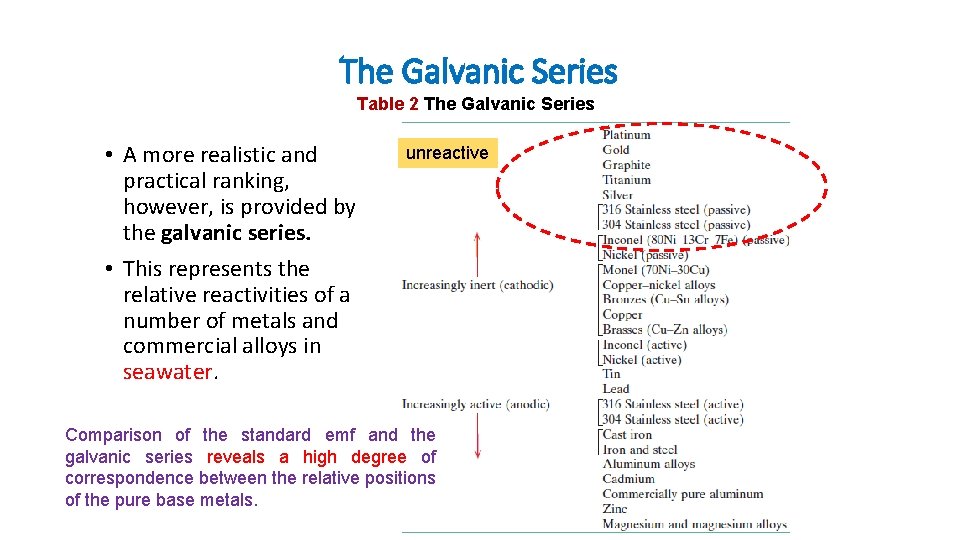
The Galvanic Series Table 2 The Galvanic Series • A more realistic and practical ranking, however, is provided by the galvanic series. • This represents the relative reactivities of a number of metals and commercial alloys in seawater. unreactive Comparison of the standard emf and the galvanic series reveals a high degree of correspondence between the relative positions of the pure base metals.

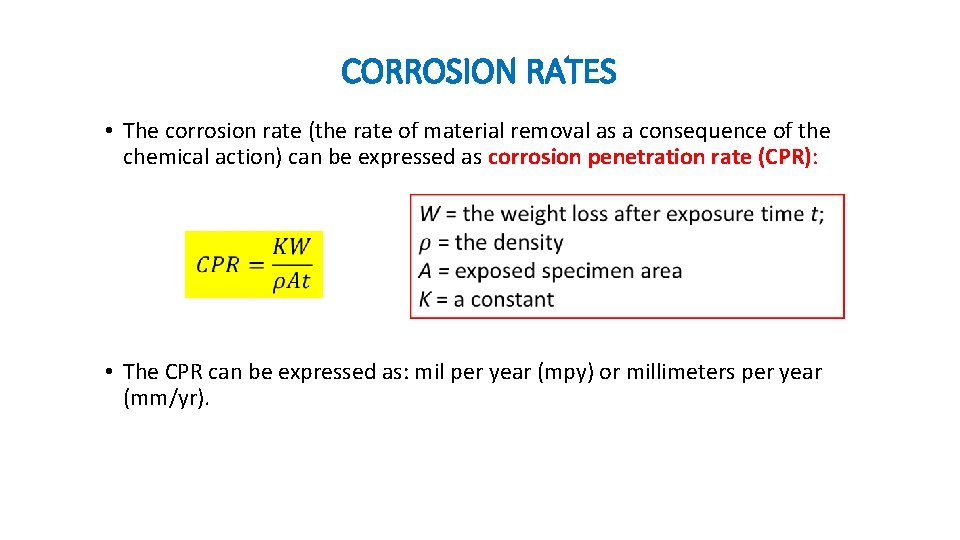
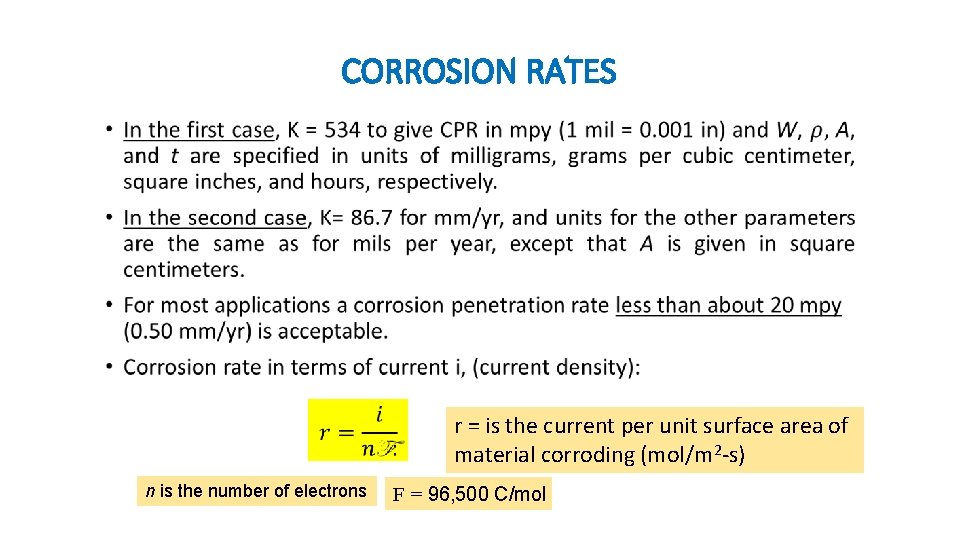
CORROSION RATES • The corrosion rate (the rate of material removal as a consequence of the chemical action) can be expressed as corrosion penetration rate (CPR): • The CPR can be expressed as: mil per year (mpy) or millimeters per year (mm/yr).

CORROSION RATES • n is the number of electrons r = is the current per unit surface area of material corroding (mol/m 2 -s) F = 96, 500 C/mol

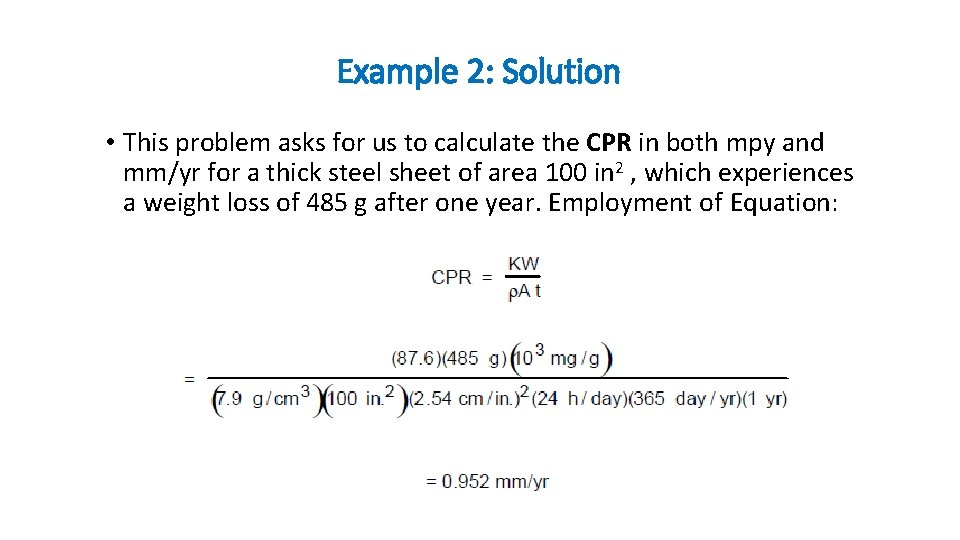
Example 2: • A thick steel sheet of area 100 in 2 is exposed to air near the ocean. After a one-year period it was found to experience a weight loss of 485 g due to corrosion. To what rate of corrosion, in both mpy and mm/yr, does this correspond?

Example 2: Solution • This problem asks for us to calculate the CPR in both mpy and mm/yr for a thick steel sheet of area 100 in 2 , which experiences a weight loss of 485 g after one year. Employment of Equation:

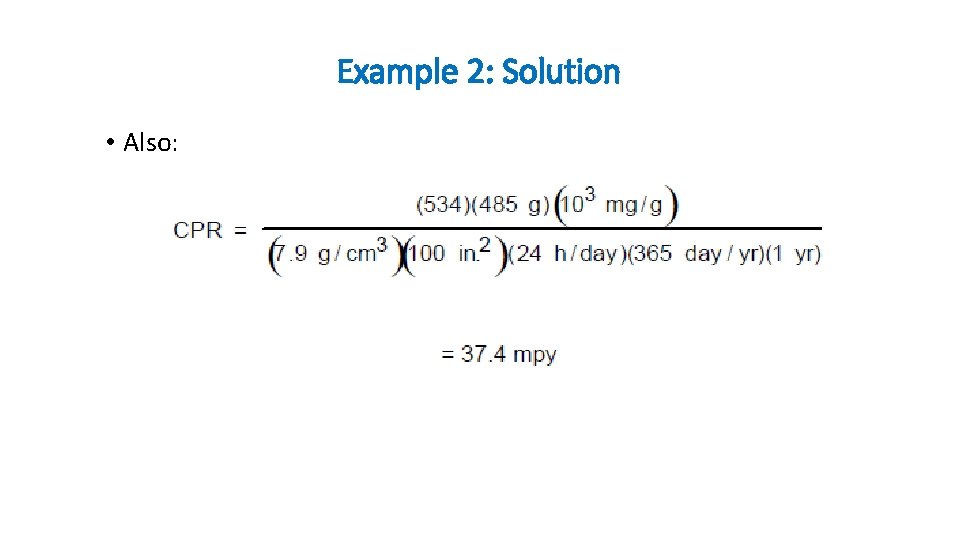
Example 2: Solution • Also:

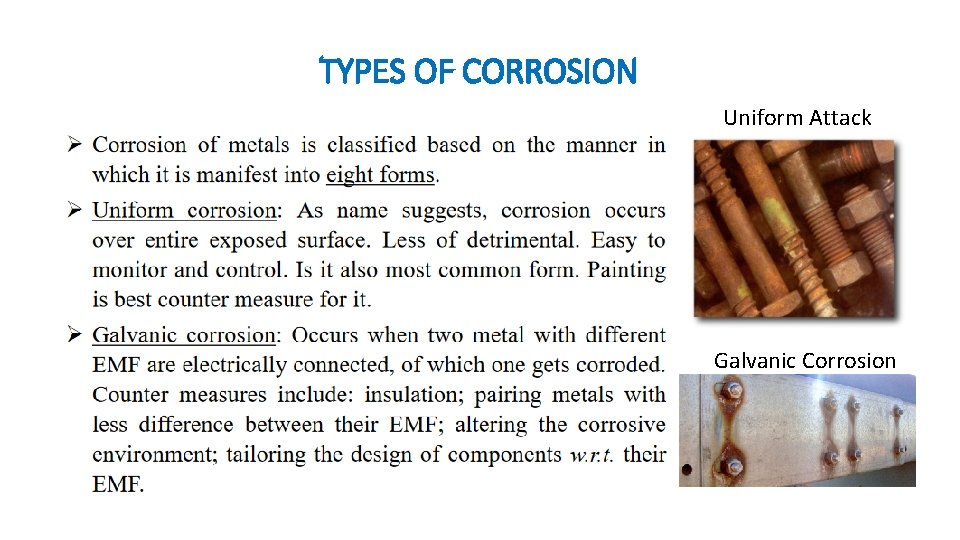
TYPES OF CORROSION Uniform Attack Galvanic Corrosion

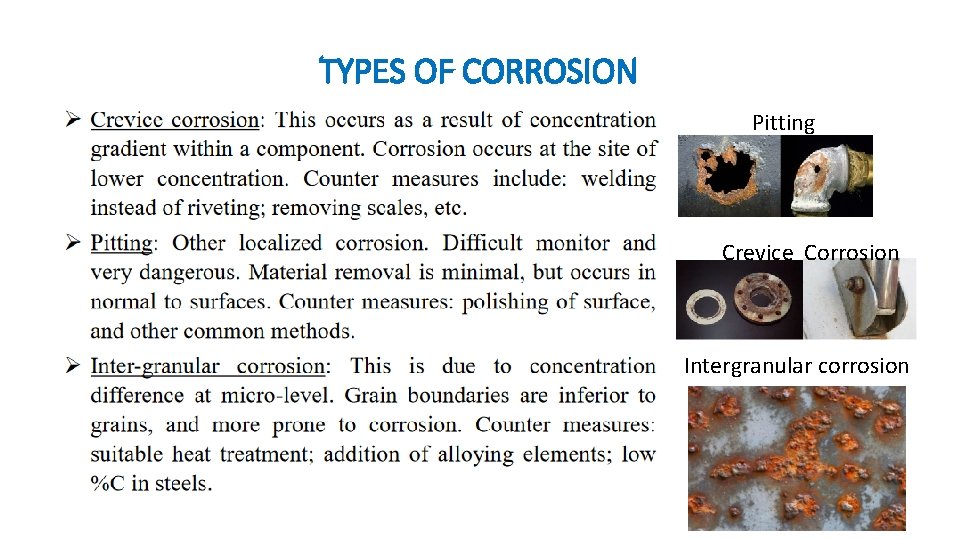
TYPES OF CORROSION Pitting Crevice Corrosion Intergranular corrosion

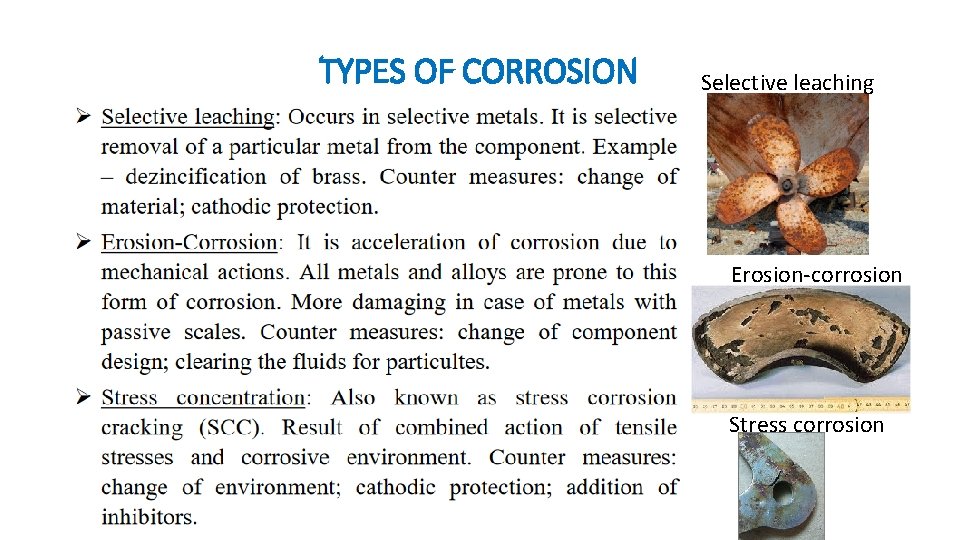
TYPES OF CORROSION Selective leaching Erosion-corrosion Stress corrosion

ENVIRONMENTAL EFFECTS • Corrosive environments include: • • the atmosphere, aqueous solutions, soils, acids, bases, inorganic solvents, molten salts, liquid metals, the human body • Atmospheric corrosion the greatest losses. • Moisture containing dissolved oxygen is the primary corrosive agent, but other substances, including sulfur compounds and sodium chloride, may also contribute • Marine atmospheres-highly corrosive because of the presence of sodium chloride.

CORROSION PREVENTION • Corrosion prevention methods include: . • • • Material selection Environmental alteration Design of materials Inhibitors Coatings Cathodic protection • Select the right and proper materials for particular corrosive environments. • The rate of corrosion directly depends upon the corrosivity of the environment and inversely proportional to the corrosion resistance of the metal

CORROSION PREVENTION Environmental Alteration • • Lowering temperature Decreasing velocity, Removing oxygen or oxidizers and Changing concentration. Design • Design consideration especially regards to galvanic and crevice corrosion and erosion–corrosion. • Besides, the design should allow for complete drainage in the case of a shutdown, and easy washing. • Since dissolved oxygen may enhance the corrosivity of many solutions, the design should, if possible, include provision for the exclusion of air.

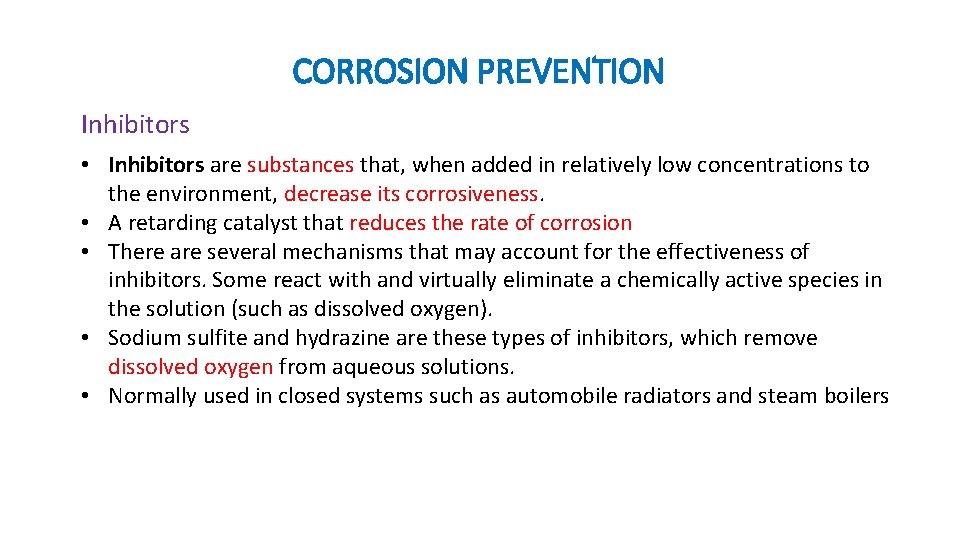
CORROSION PREVENTION Inhibitors • Inhibitors are substances that, when added in relatively low concentrations to the environment, decrease its corrosiveness. • A retarding catalyst that reduces the rate of corrosion • There are several mechanisms that may account for the effectiveness of inhibitors. Some react with and virtually eliminate a chemically active species in the solution (such as dissolved oxygen). • Sodium sulfite and hydrazine are these types of inhibitors, which remove dissolved oxygen from aqueous solutions. • Normally used in closed systems such as automobile radiators and steam boilers

CORROSION PREVENTION Coatings • The most common method of protection is simply painting the metal object. However, coatings such as concrete, plastic (PVC), fibreglass, rubber and bitumen can also be used. • They all simply stop the oxygen and/or the water from coming into contact with the metal. • The main problem with this method is if you scratch the protective coating. The iron will begin to corrode and will lift off even more of the protective surface.

CORROSION PREVENTION Cathodic protection • Involves supplying, from an external source, electrons to the metal to be protected, making it a cathode; the reaction above is thus forced in the reverse (or reduction) direction.

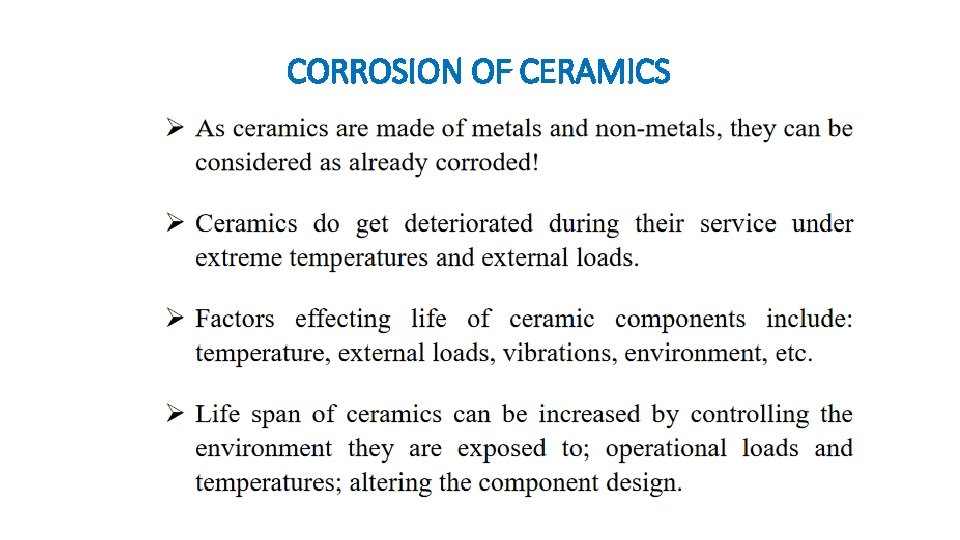
CORROSION OF CERAMICS

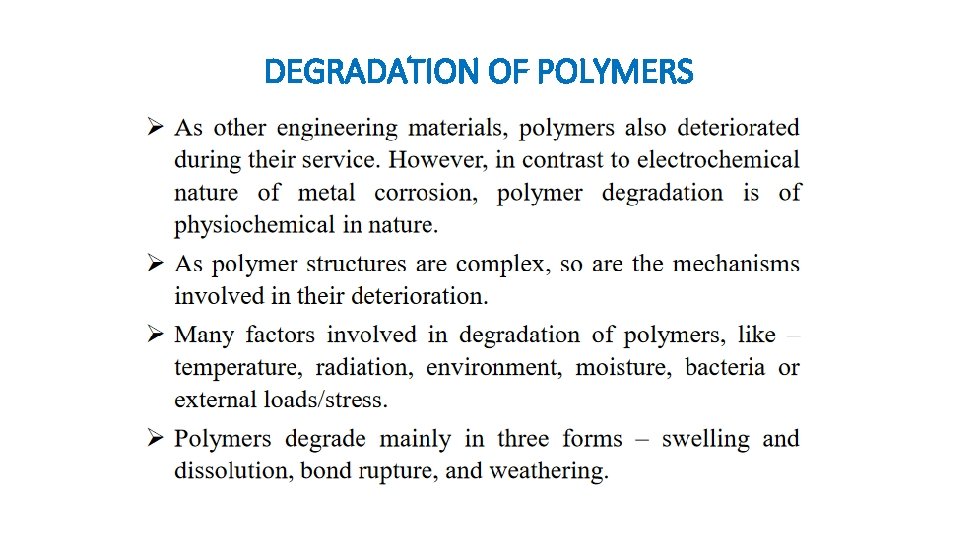
DEGRADATION OF POLYMERS

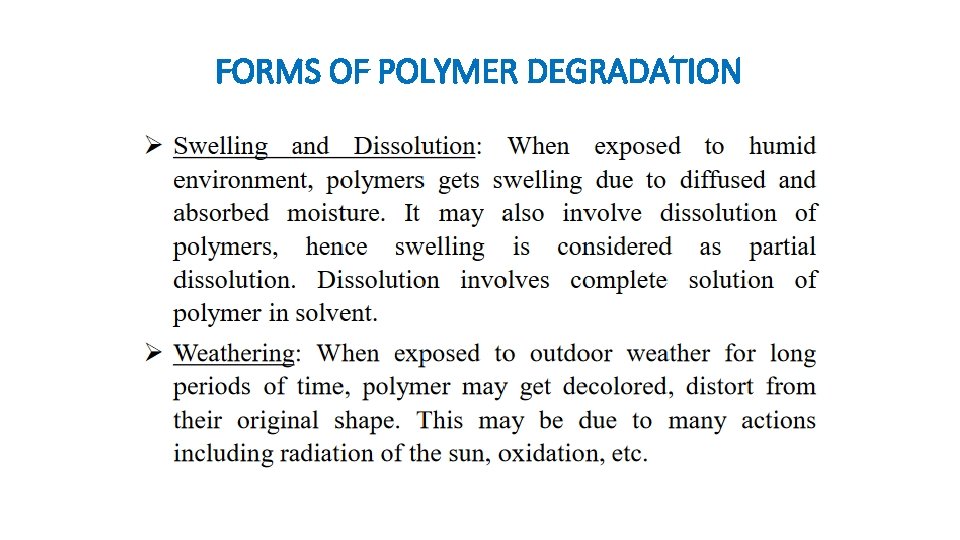
FORMS OF POLYMER DEGRADATION

FORMS OF POLYMER DEGRADATION