Engineering Alloys 307 Lecture 7 Titanium Alloys I

- Slides: 15

Engineering Alloys (307) Lecture 7 Titanium Alloys I David Dye Department of Materials, Imperial College Royal School of Mines, Prince Consort Road, London SW 7 2 BP, UK +44 (207) 594 -6811, david. dye@imperial. ac. uk © Imperial College London

Outline 2 © Imperial College London • • • Ti primary production CP Ti and applications α-Ti alloying, alloy design near-α alloy microstructures, forging and heat treatment α/β alloys, Ti-6 Al-4 V defects

Ti Primary Production – Kroll Process 3 © Imperial College London • Ti common in Earth’s crust • Energy to separate ~125 MWhr/tonne (£ 4/kg just in power) • Batch process over 5 days: – – – Produce Ti. Cl 4 from Ti. O 2 and Cl 2 Ti. Cl 4 + 2 Mg → 2 Mg. Cl 2 + Ti chip out Ti sponge (5 -8 t) from reactor cost £ 5/kg Chlorides corrosive, nasty • World annual capacity ~100, 000 t, demand ~60, 000 t ($500 m - small) • Need a cheaper process that is direct – FFC (Cambridge) and others

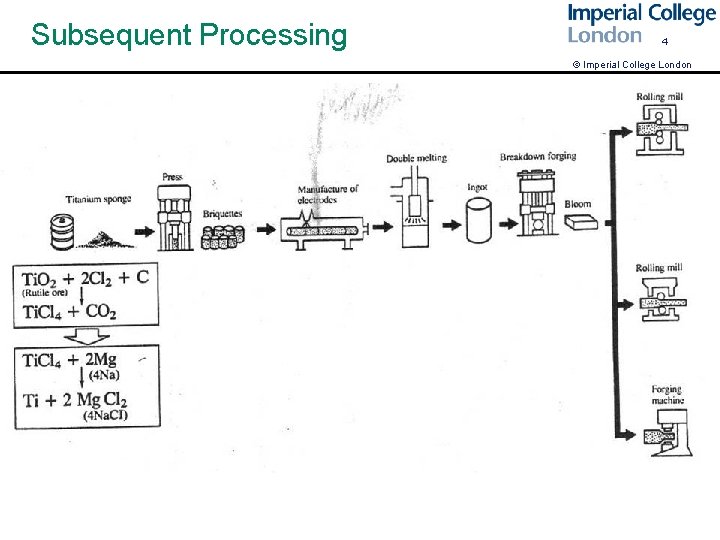
Subsequent Processing 4 © Imperial College London harvey fig p 11

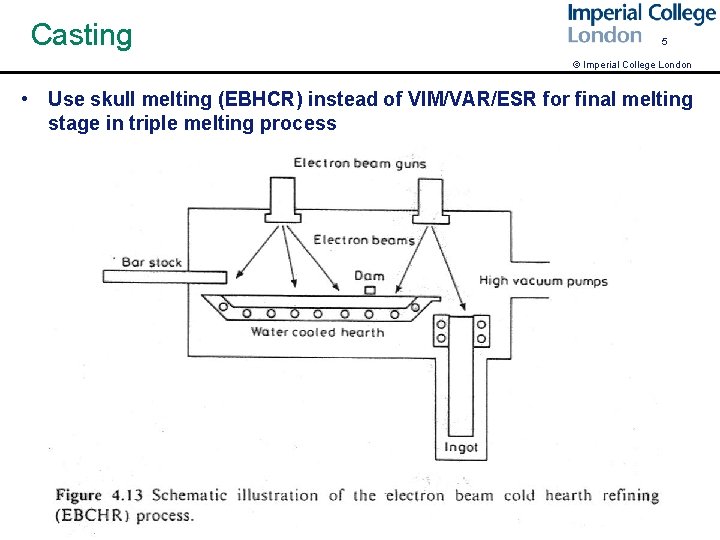
Casting 5 © Imperial College London • Use skull melting (EBHCR) instead of VIM/VAR/ESR for final melting stage in triple melting process

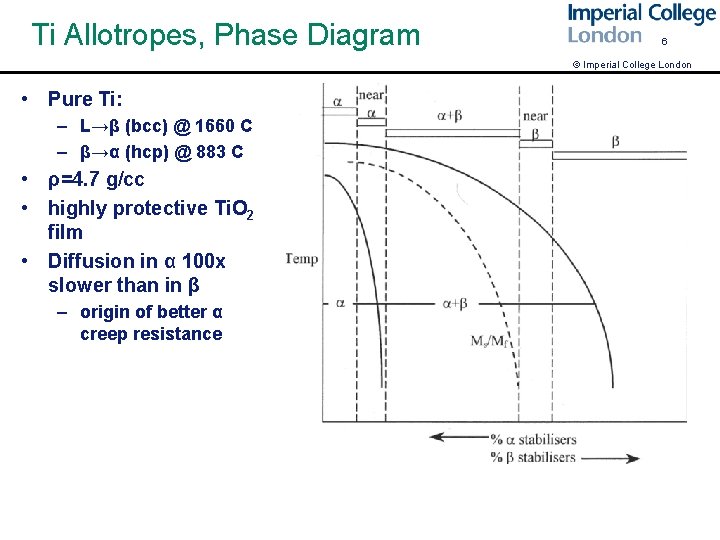
Ti Allotropes, Phase Diagram 6 © Imperial College London • Pure Ti: – L→β (bcc) @ 1660 C – β→α (hcp) @ 883 C • ρ=4. 7 g/cc • highly protective Ti. O 2 film • Diffusion in α 100 x slower than in β – origin of better α creep resistance

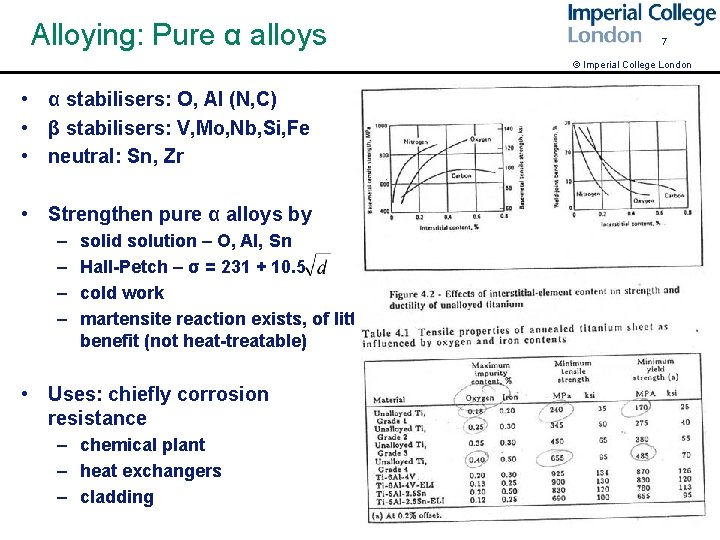
Alloying: Pure α alloys 7 © Imperial College London • α stabilisers: O, Al (N, C) • β stabilisers: V, Mo, Nb, Si, Fe • neutral: Sn, Zr • Strengthen pure α alloys by – – solid solution – O, Al, Sn Hall-Petch – σ = 231 + 10. 5 cold work martensite reaction exists, of little benefit (not heat-treatable) • Uses: chiefly corrosion resistance – chemical plant – heat exchangers – cladding harvey fig p 13 Table of CP Ti

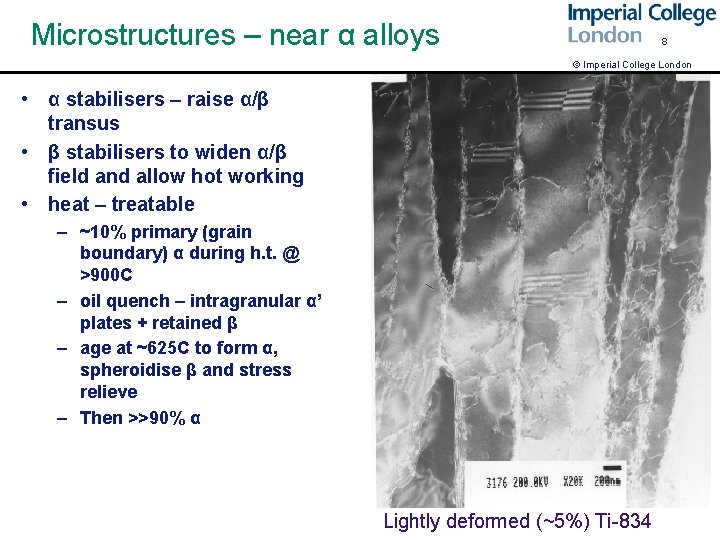
Microstructures – near α alloys 8 © Imperial College London • α stabilisers – raise α/β transus • β stabilisers to widen α/β field and allow hot working • heat – treatable – ~10% primary (grain boundary) α during h. t. @ >900 C – oil quench – intragranular α’ plates + retained β – age at ~625 C to form α, spheroidise β and stress relieve – Then >>90% α Lightly deformed (~5%) Ti-834

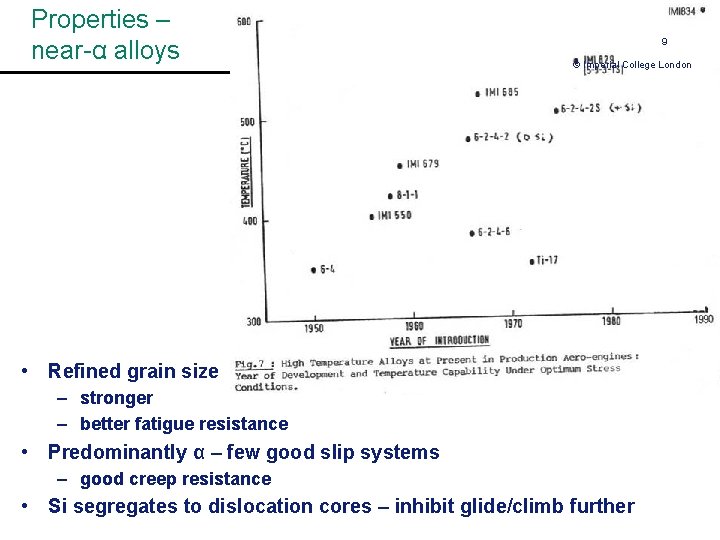
Properties – near-α alloys 9 © Imperial College London • Refined grain size – stronger – better fatigue resistance • Predominantly α – few good slip systems – good creep resistance • Si segregates to dislocation cores – inhibit glide/climb further

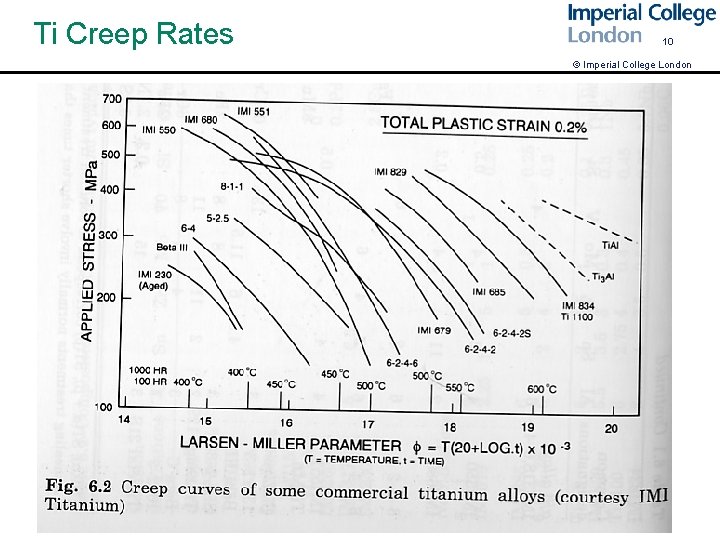
Ti Creep Rates 10 © Imperial College London

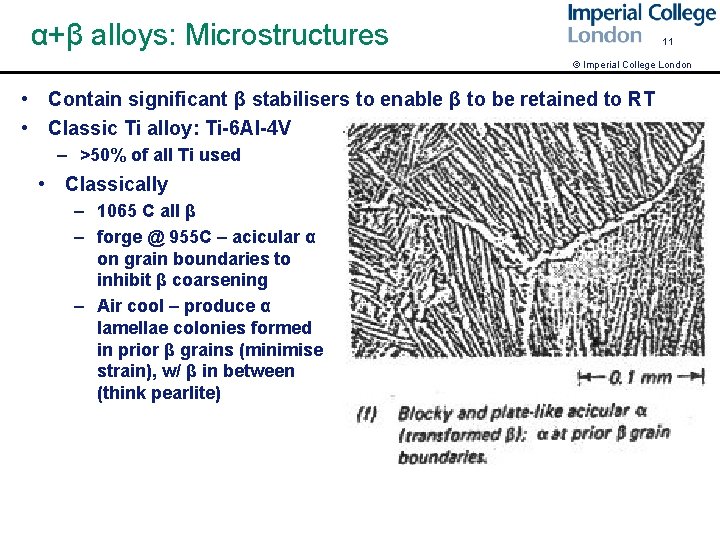
α+β alloys: Microstructures 11 © Imperial College London • Contain significant β stabilisers to enable β to be retained to RT • Classic Ti alloy: Ti-6 Al-4 V – >50% of all Ti used • Classically – 1065 C all β – forge @ 955 C – acicular α on grain boundaries to inhibit β coarsening – Air cool – produce α lamellae colonies formed in prior β grains (minimise strain), w/ β in between (think pearlite)

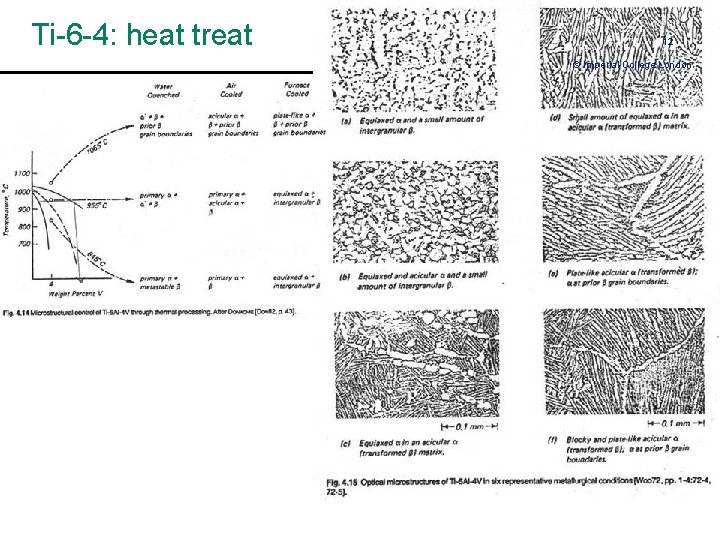
Ti-6 -4: heat treat 12 © Imperial College London

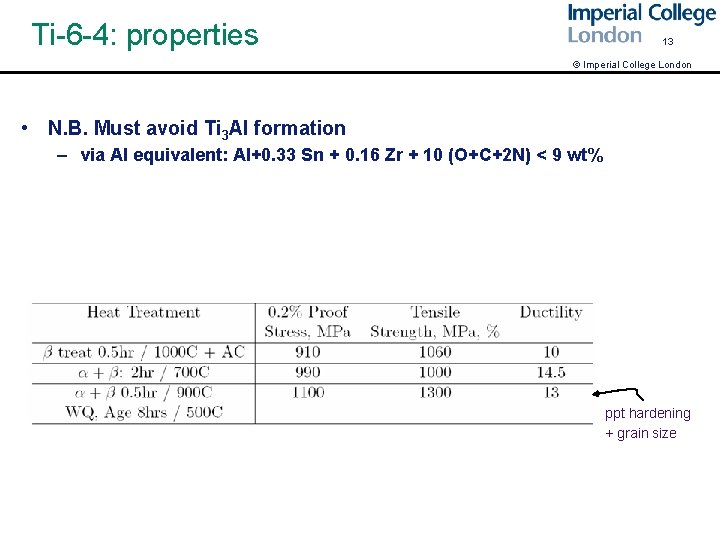
Ti-6 -4: properties 13 © Imperial College London • N. B. Must avoid Ti 3 Al formation – via Al equivalent: Al+0. 33 Sn + 0. 16 Zr + 10 (O+C+2 N) < 9 wt% ppt hardening + grain size

Defects 14 © Imperial College London • Major α-related problem is the production of α-rich regions due to oxygen (+N) embrittlement – the entrapment of O-rich particles during melting • Called α case • Also a problem in welding – often Ti is welded in an Ar-filled cavity to avoid this • β alloys suffer from β-rich regions from solute segregation (β flecks), and/or from embrittling ω phase, a diffusionless way to transform from β-bcc to a hexagonal phase. – more in lecture on β alloys

Review: Titanium I (L 7) 15 © Imperial College London near-α microstructure α/β microstructure Casting α-Ti Alloys Phase Diagram