Energy Review Remember Thermal energy the sum of


Energy Review Remember… • Thermal energy: the sum of the kinetic and potential energy of particles in an object – Kinetic energy: energy in motion – Potential energy: stored energy • This relates to matter – specifically how matter physically changes states due to the motion of the particles within it. • A theory explains what we know to be true about this.
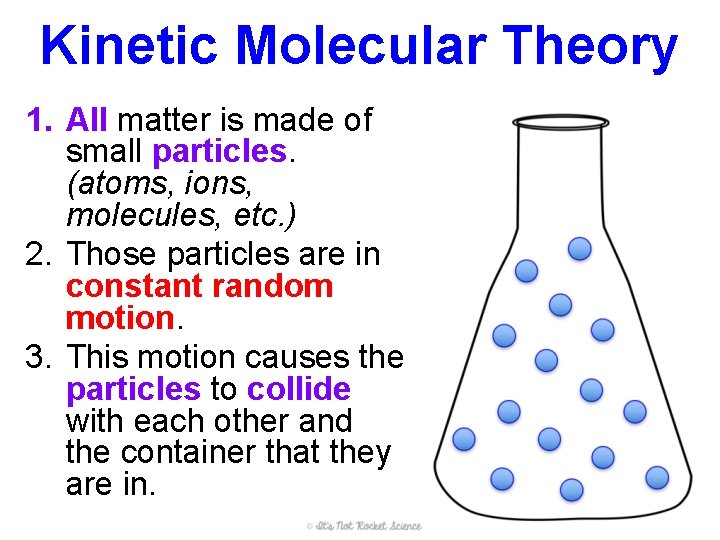
Kinetic Molecular Theory 1. All matter is made of small particles. (atoms, ions, molecules, etc. ) 2. Those particles are in constant random motion. 3. This motion causes the particles to collide with each other and the container that they are in.
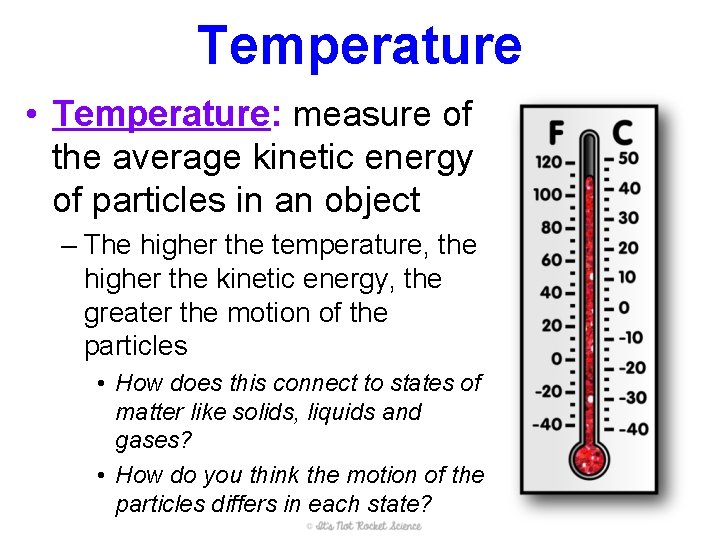
Temperature • Temperature: measure of the average kinetic energy of particles in an object – The higher the temperature, the higher the kinetic energy, the greater the motion of the particles • How does this connect to states of matter like solids, liquids and gases? • How do you think the motion of the particles differs in each state?
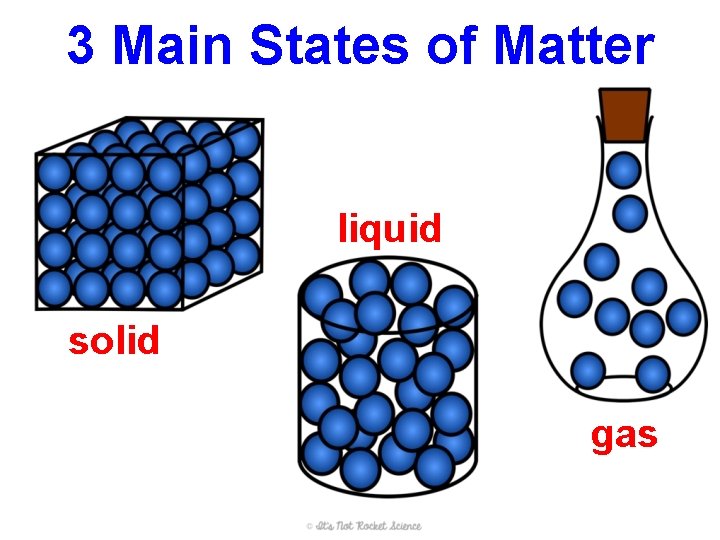
3 Main States of Matter liquid solid gas
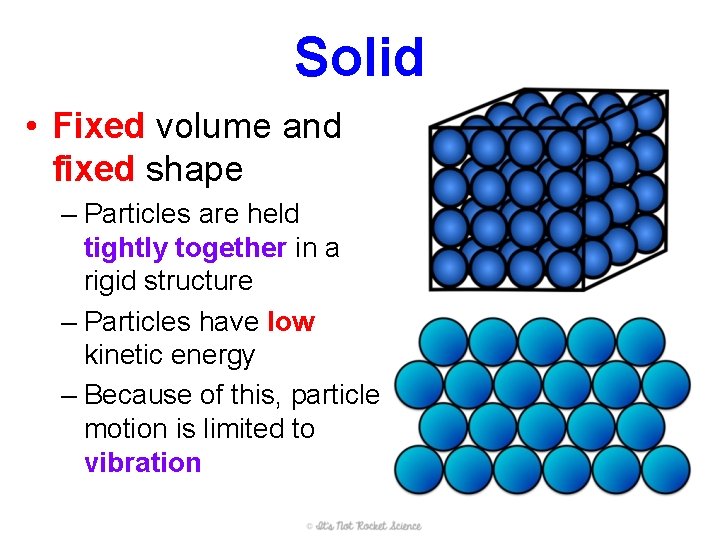
Solid • Fixed volume and fixed shape – Particles are held tightly together in a rigid structure – Particles have low kinetic energy – Because of this, particle motion is limited to vibration
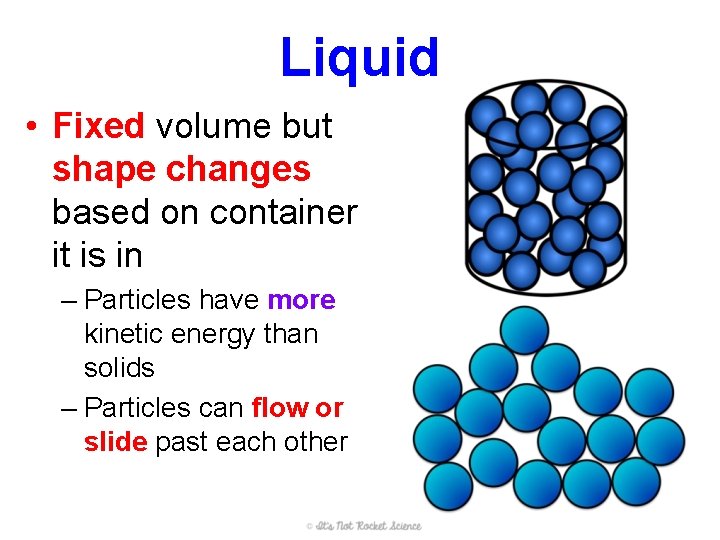
Liquid • Fixed volume but shape changes based on container it is in – Particles have more kinetic energy than solids – Particles can flow or slide past each other
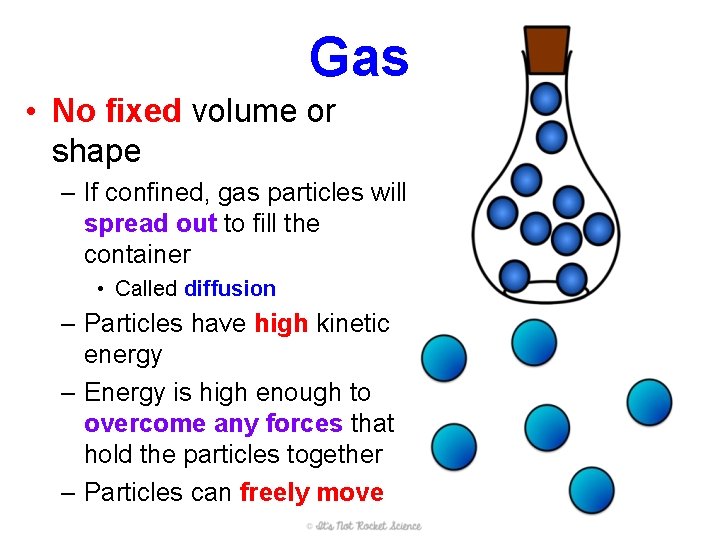
Gas • No fixed volume or shape – If confined, gas particles will spread out to fill the container • Called diffusion – Particles have high kinetic energy – Energy is high enough to overcome any forces that hold the particles together – Particles can freely move

2 other States of Matter We won’t focus as much on, but are worth mentioning • Plasma: matter composed of positively and negatively charged particles with extremely high kinetic energy – Most common form of matter in the universe – Makes up stars, neon lights, auroras

2 other States of Matter We won’t focus as much on, but are worth mentioning • Bose-Einstein condensates: atoms that are super-cooled to such a low temperature (almost absolute zero) that they form a “super atom” – Created by scientists in 1995 – All particles in the super atom are at the same energetic state – Will even slow down light that passes through – Used to simulate conditions in a black hole

• https: //www. youtube. com/watch? v=u 8 w. NSV x. YZGI
Changing States • When particles gain or lose thermal energy, they can undergo a state change – This is a physical change because the identity of the matter is still the same • Ex. When ice melts it is still water (H 2 O) • Heat of fusion: the amount of energy needed to turn a solid into a liquid at its melting point – When heat is added to a solid, it has more kinetic energy so the particles vibrate faster and start moving farther apart as they transition to the liquid state • Heat of vaporization: the amount of energy needed to turn a liquid into a gas at its boiling point
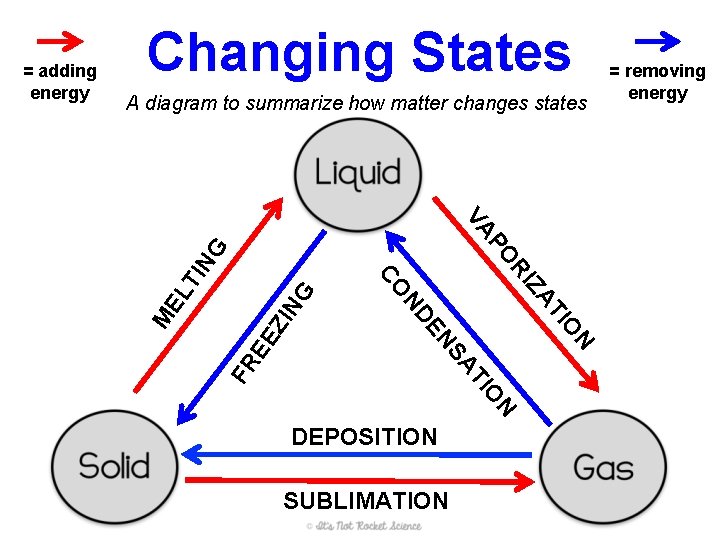
Changing States A diagram to summarize how matter changes states NG TI ZI FR N O DEPOSITION SUBLIMATION N IO TI SA EN EE AT ND EL Z RI NG PO VA CO M = adding energy = removing energy
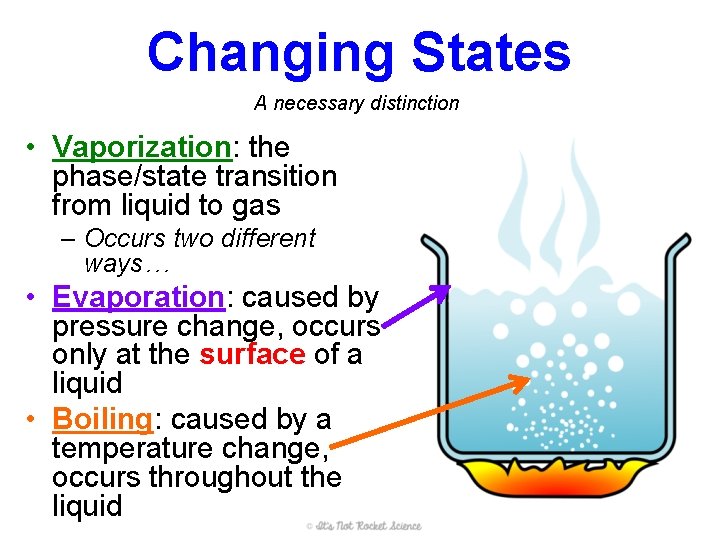
Changing States A necessary distinction • Vaporization: the phase/state transition from liquid to gas – Occurs two different ways… • Evaporation: caused by pressure change, occurs only at the surface of a liquid • Boiling: caused by a temperature change, occurs throughout the liquid
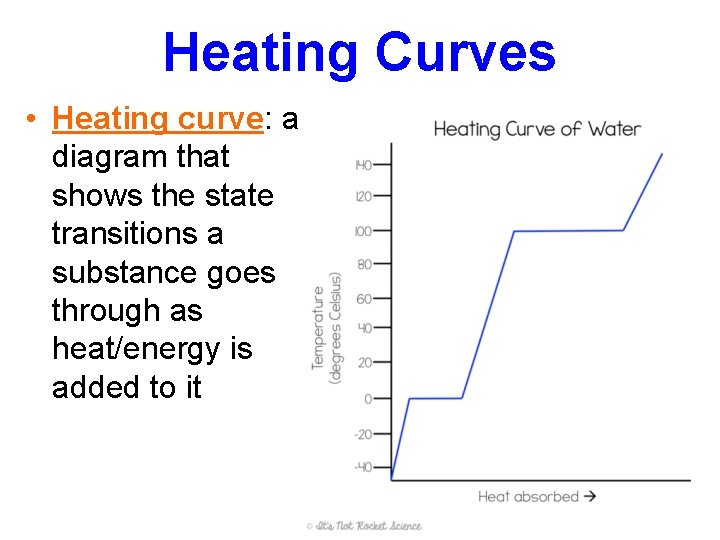
Heating Curves • Heating curve: a diagram that shows the state transitions a substance goes through as heat/energy is added to it
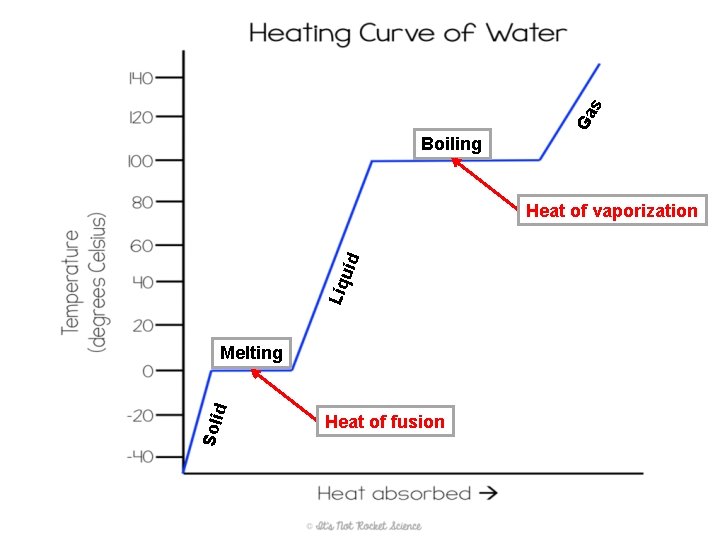
Ga s Boiling Liq uid Heat of vaporization Soli d Melting Heat of fusion
- Slides: 16