Energy Physical Changes Transfer vs Transformation Using a





- Slides: 8

Energy – Physical Changes

Transfer vs. Transformation • Using a burning log as an example, describe the difference between: ▫ Energy transformation: is the changing of energy from one form to another log (chemical E) + oxygen (O 2) radiant E + thermal E Combustion reaction ▫ Energy transfer: the movement of energy from one place to another • Ex: transfer of thermal energy (heat) from the burning log to people Transfer of thermal energy occurs from a place with the highest temperature to a place with the lowest temperature

What do you think? • Sun`s radiant energy shining on a plant ▫ Transfer • Chemical energy of an apple in relation to the human eating it ▫ Transformation • Mechanical energy of falling water on a water wheel ▫ Transfer

Physical Changes • A physical change through energy, causes a physical change does not affect the nature or the characteristic properties of matter ▫ Types: �Phase change �Dissolution �Deformation

Make a list of verbs that represent physical changes Examples include: cutting, grinding, bending, the melting of the metal gallium, the freezing of water and the condensation of steam to water. Boil, freeze, dissolve, melt, condense, break, split, crack, grind, cut, crush and bend.

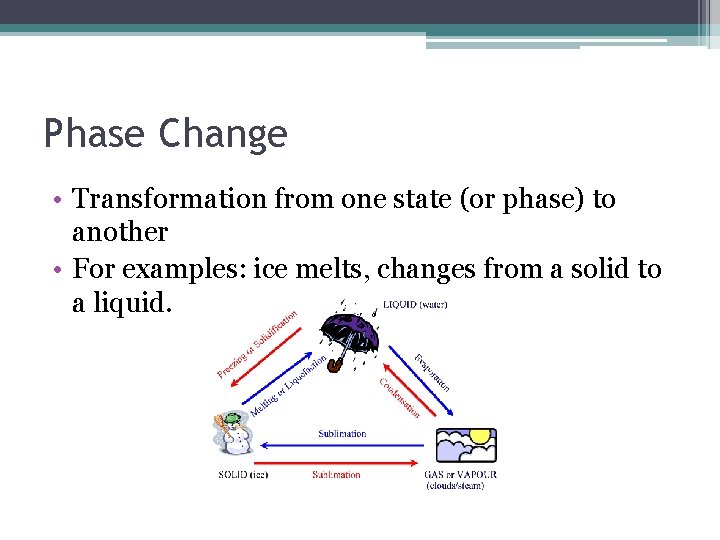
Phase Change • Transformation from one state (or phase) to another • For examples: ice melts, changes from a solid to a liquid.

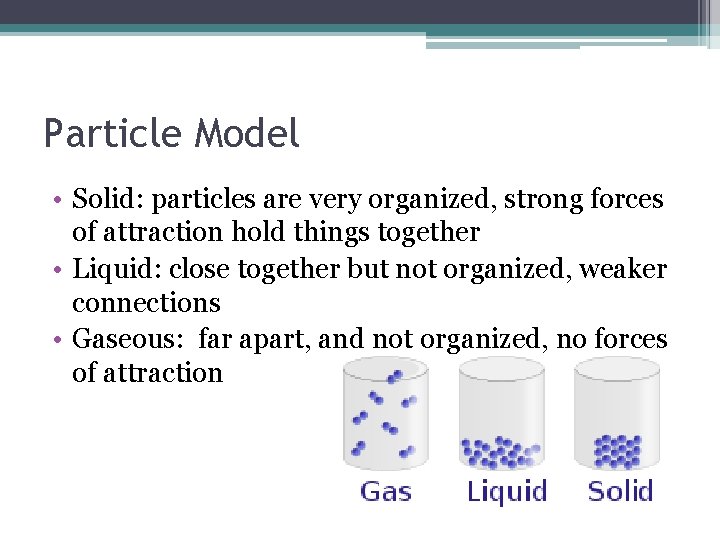
Particle Model • Solid: particles are very organized, strong forces of attraction hold things together • Liquid: close together but not organized, weaker connections • Gaseous: far apart, and not organized, no forces of attraction

Absorb or release • Liquid water + energy water vapour ▫ Transformation of liquid to vapour absorbs energy �The water particles absorb the energy from the heat source making them rapid then using that energy to change to vapour • Liquid water ice + energy ▫ Transformation of liquid water into ice releases energy