Energy Levels Sublevels Orbitals Electron Review Exact location

Energy Levels, Sublevels & Orbitals

Electron Review • Exact location unknown • Found outside of the nucleus in electron cloud • Found in energy levels – Energy levels closer to the nucleus have less energy than those farther from the nucleus – Electrons fill the energy levels closest to the nucleus first
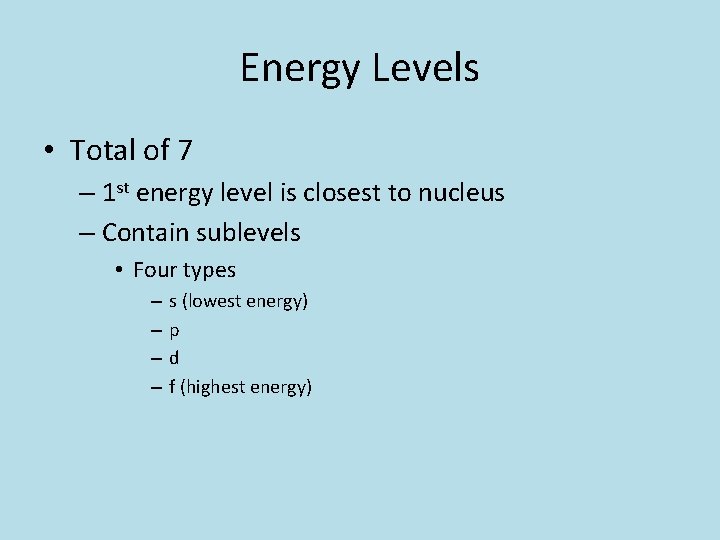
Energy Levels • Total of 7 – 1 st energy level is closest to nucleus – Contain sublevels • Four types – – s (lowest energy) p d f (highest energy)

– Each energy level does not contain the same sublevels – As the distance from the nucleus increases • energy levels can hold more electrons – Therefore, they can have more sublevels
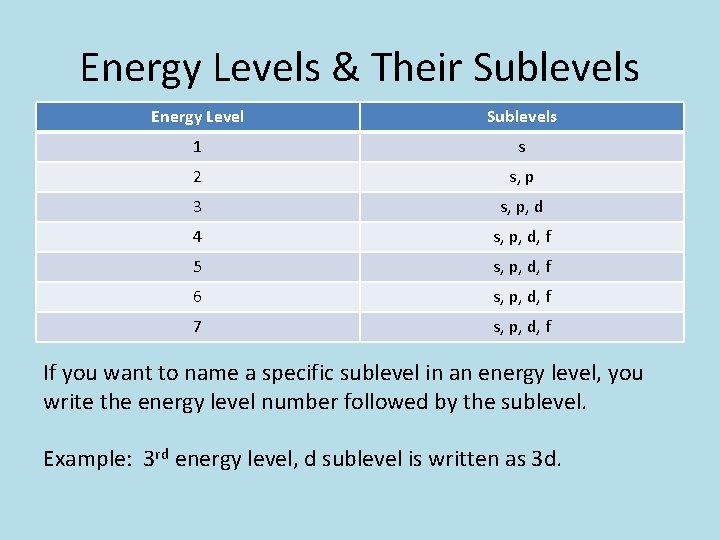
Energy Levels & Their Sublevels Energy Level Sublevels 1 s 2 s, p 3 s, p, d 4 s, p, d, f 5 s, p, d, f 6 s, p, d, f 7 s, p, d, f If you want to name a specific sublevel in an energy level, you write the energy level number followed by the sublevel. Example: 3 rd energy level, d sublevel is written as 3 d.
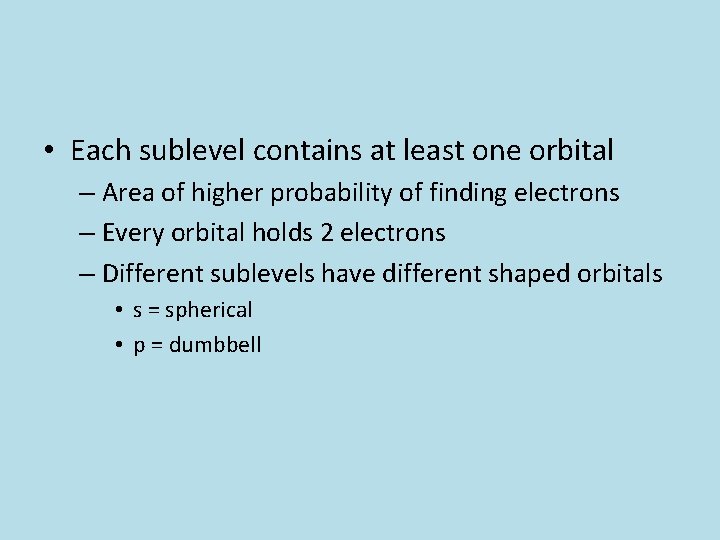
• Each sublevel contains at least one orbital – Area of higher probability of finding electrons – Every orbital holds 2 electrons – Different sublevels have different shaped orbitals • s = spherical • p = dumbbell
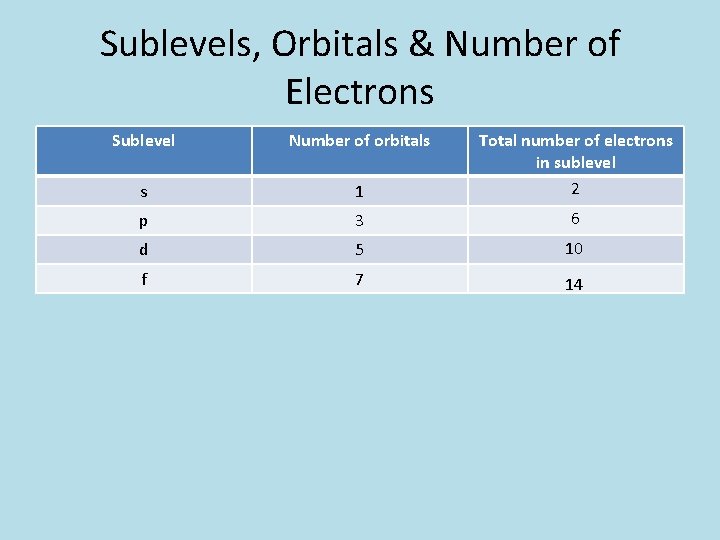
Sublevels, Orbitals & Number of Electrons Sublevel Number of orbitals s 1 Total number of electrons in sublevel 2 p 3 6 d 5 10 f 7 14

Energy Levels and Electrons Energy Level Total Number of Electrons 1 2 2 2+6=8 3 4 2 + 6 + 10 = 18 2 + 6 +10 + 14 = 32 5 32 6 32
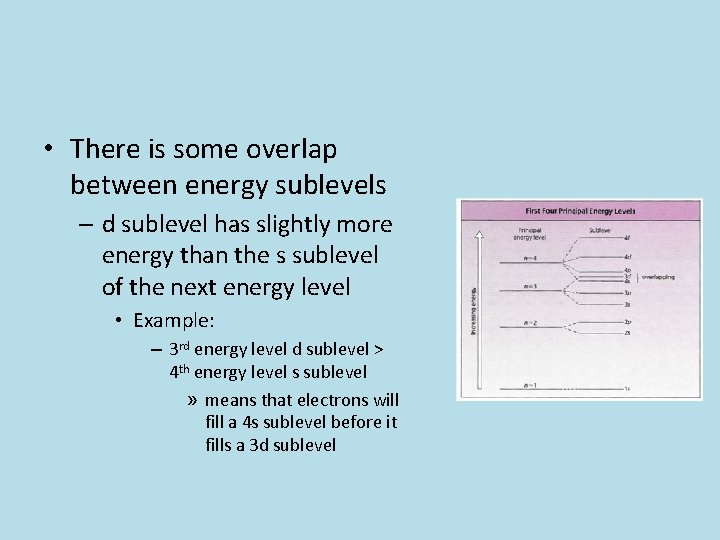
• There is some overlap between energy sublevels – d sublevel has slightly more energy than the s sublevel of the next energy level • Example: – 3 rd energy level d sublevel > 4 th energy level s sublevel » means that electrons will fill a 4 s sublevel before it fills a 3 d sublevel
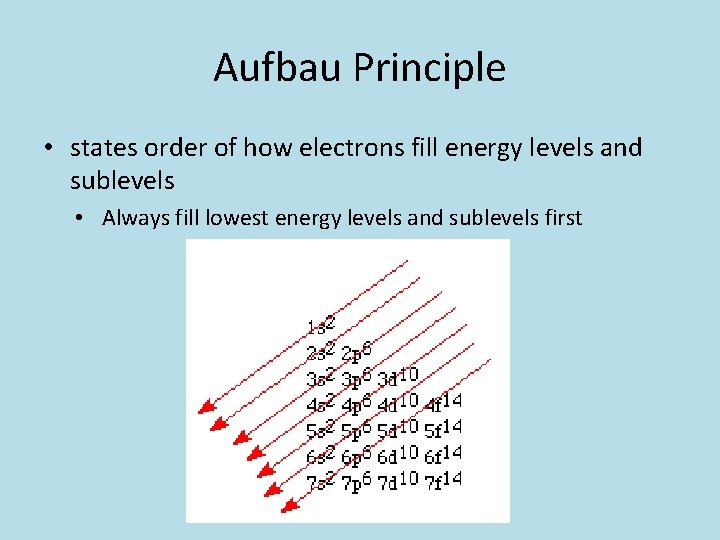
Aufbau Principle • states order of how electrons fill energy levels and sublevels • Always fill lowest energy levels and sublevels first
- Slides: 10