Energy in Earth Processes Earth Science Mr Cloud




- Slides: 18

Energy in Earth Processes Earth Science Mr. Cloud

Energy • • - Energy: is the capacity or ability to do work. The capacity to make a change. Energy comes in many forms: Chemical Energy Radiant Energy Solar Energy Thermal Energy Mechanical Energy Nuclear Energy Electrical Energy is expressed in units of work Joules (k. J, J) or Calories (kcal, cal)

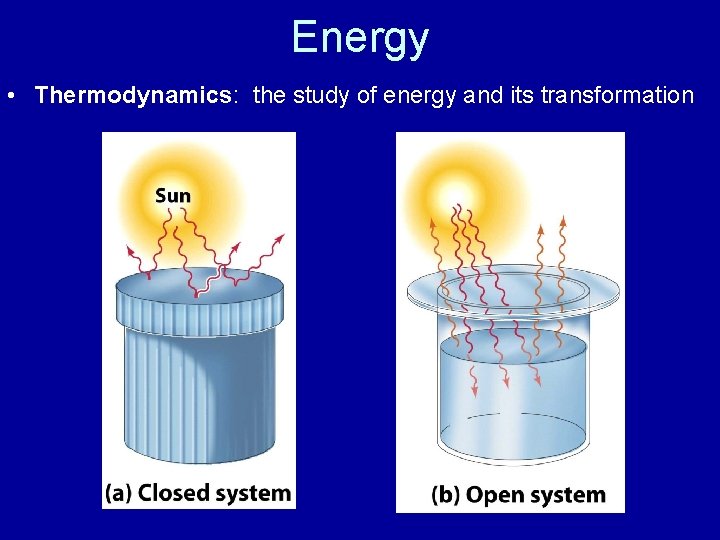
Energy • Thermodynamics: the study of energy and its transformation

Energy • 1 st Law of Thermodynamics – • Law of Conservation of Total Energy • energy can change forms, but is not created or destroyed • Where does the energy to light your home come from? • Using you knowledge of the 1 st Law of Thermodynamics, arrange the following forms of energy in the proper order. Discuss how one form transforms into another.

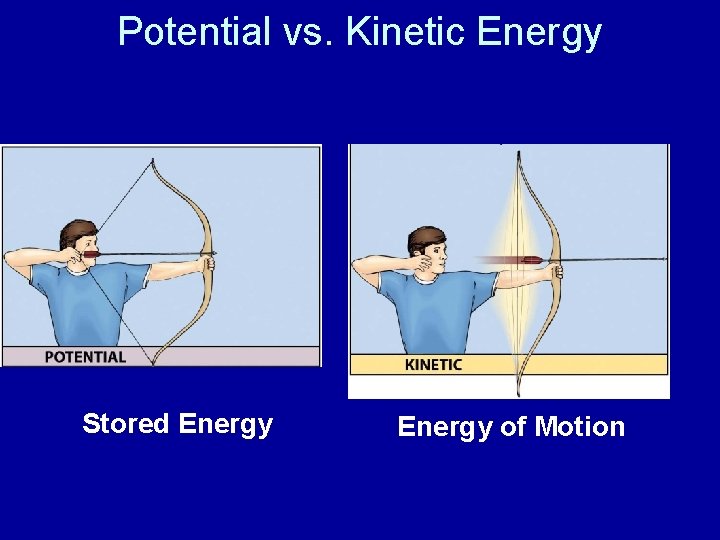
Potential vs. Kinetic Energy Stored Energy of Motion

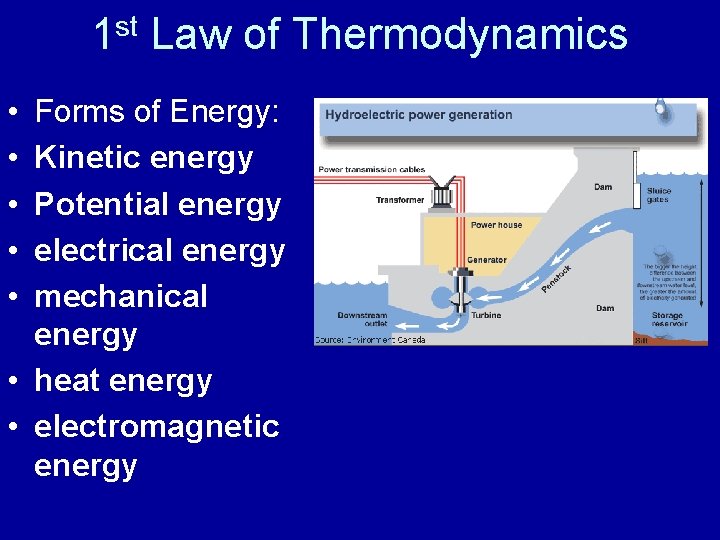
1 st Law of Thermodynamics • • • Forms of Energy: Kinetic energy Potential energy electrical energy mechanical energy • heat energy • electromagnetic energy


Temperature v. Heat What is the difference between temperature and heat? Use your review books to figure it out. 2009 Edition: Topic 5

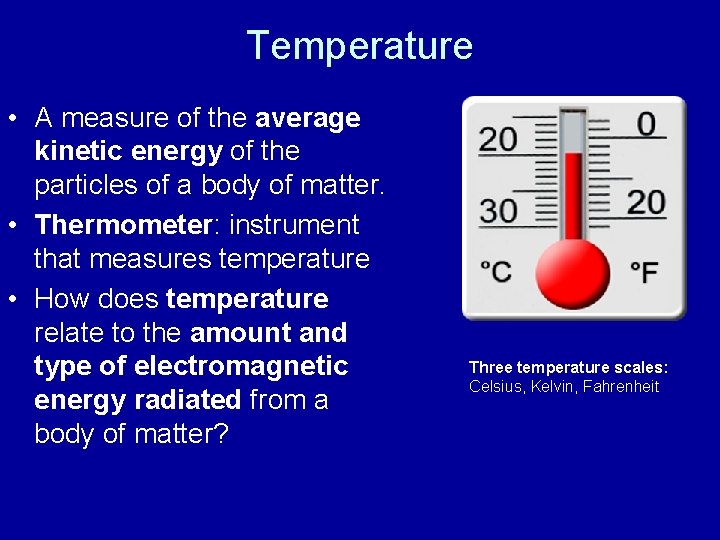
Temperature • A measure of the average kinetic energy of the particles of a body of matter. • Thermometer: instrument that measures temperature • How does temperature relate to the amount and type of electromagnetic energy radiated from a body of matter? Three temperature scales: Celsius, Kelvin, Fahrenheit

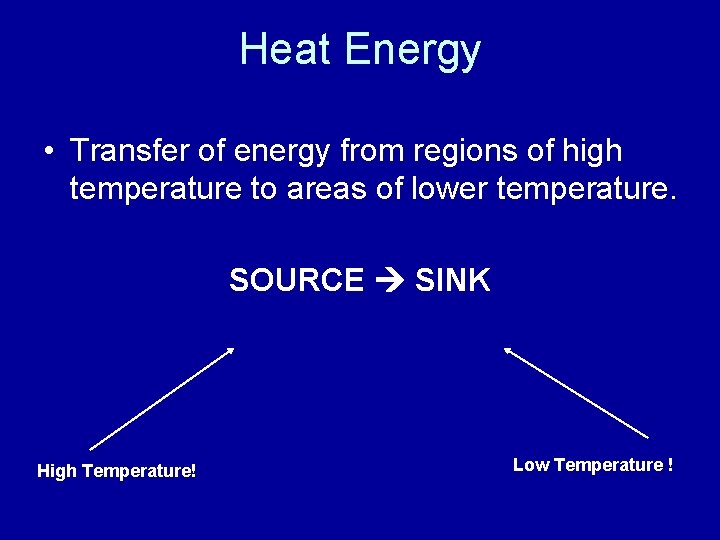
Heat Energy • Transfer of energy from regions of high temperature to areas of lower temperature. SOURCE SINK High Temperature! Low Temperature !


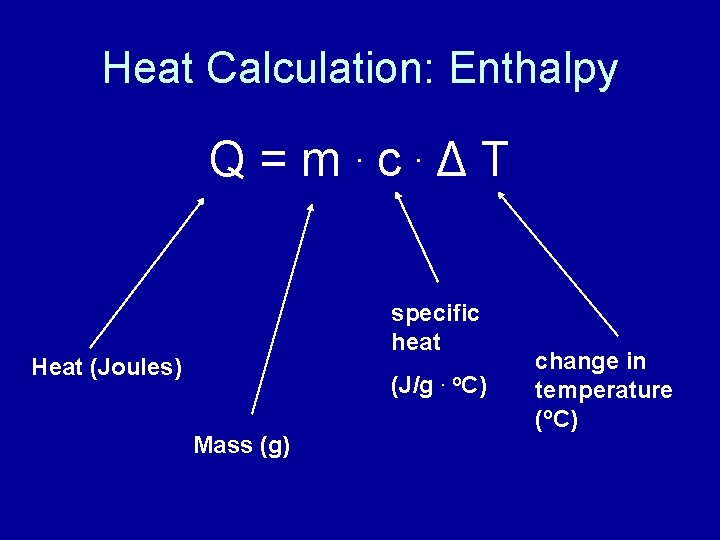
Heat Calculation: Enthalpy Q=m c ΔT. . specific heat Heat (Joules) (J/g. o. C) Mass (g) change in temperature (o. C)

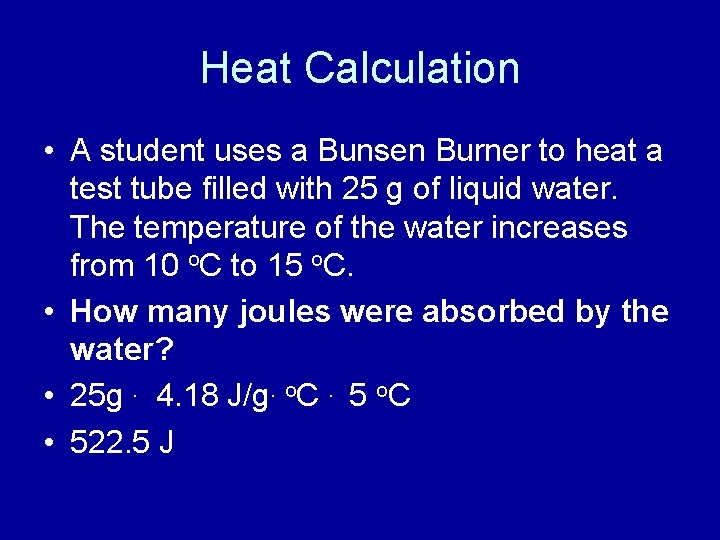
Heat Calculation • A student uses a Bunsen Burner to heat a test tube filled with 25 g of liquid water. The temperature of the water increases from 10 o. C to 15 o. C. • How many joules were absorbed by the water? • 25 g. 4. 18 J/g. o. C. 5 o. C • 522. 5 J

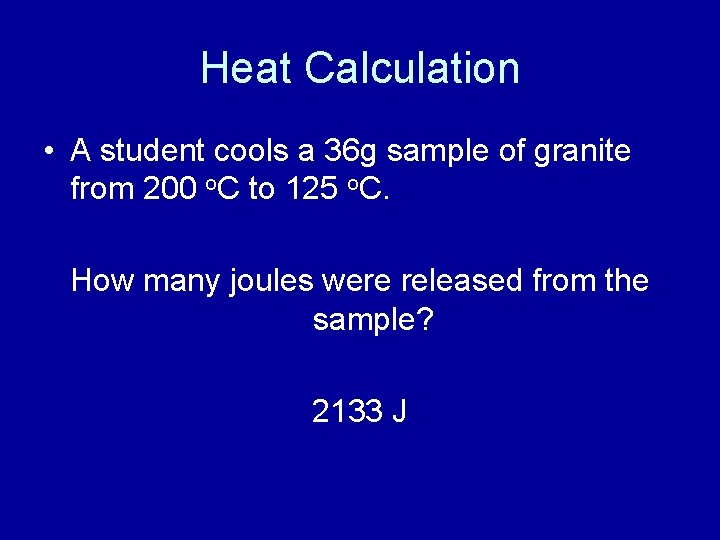
Heat Calculation • A student cools a 36 g sample of granite from 200 o. C to 125 o. C. How many joules were released from the sample? 2133 J

Types of Energy Transfer 1. Conduction 2. Convection 3. Radiation

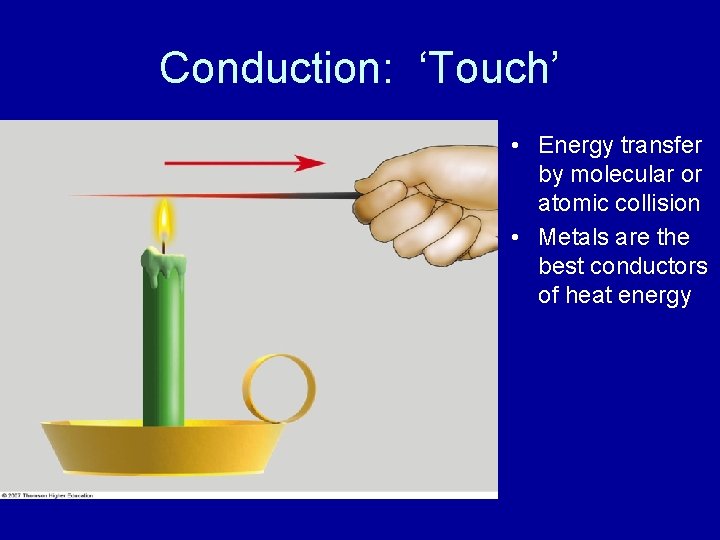
Conduction: ‘Touch’ • Energy transfer by molecular or atomic collision • Metals are the best conductors of heat energy

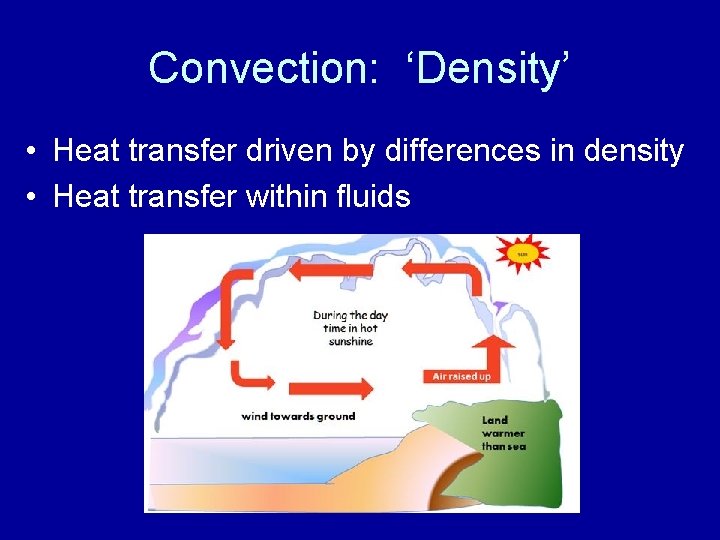
Convection: ‘Density’ • Heat transfer driven by differences in density • Heat transfer within fluids

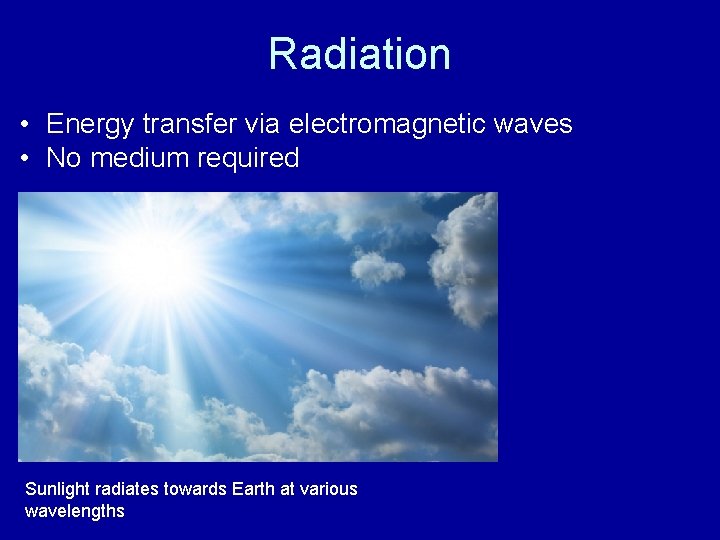
Radiation • Energy transfer via electromagnetic waves • No medium required Sunlight radiates towards Earth at various wavelengths