Energy Flow Through Cells Energy n What are



- Slides: 20

Energy Flow Through Cells

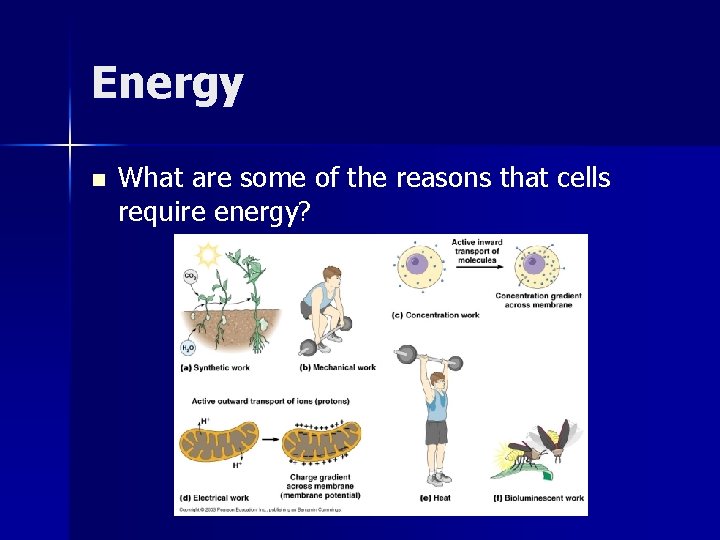
Energy n What are some of the reasons that cells require energy?

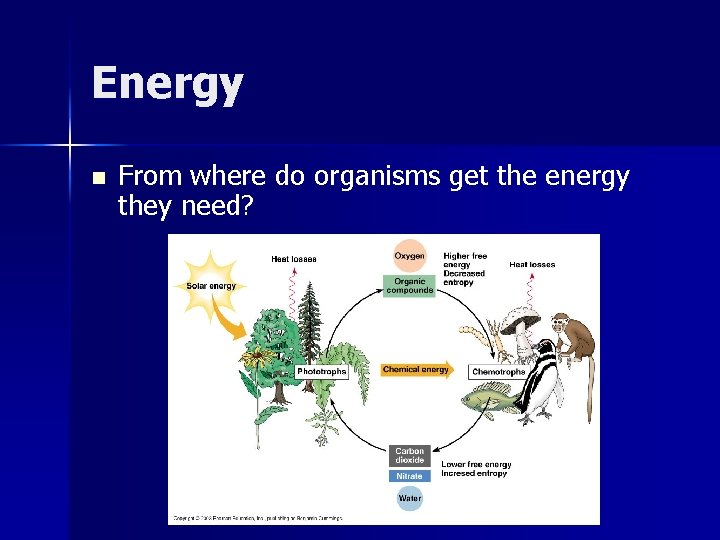
Energy n From where do organisms get the energy they need?

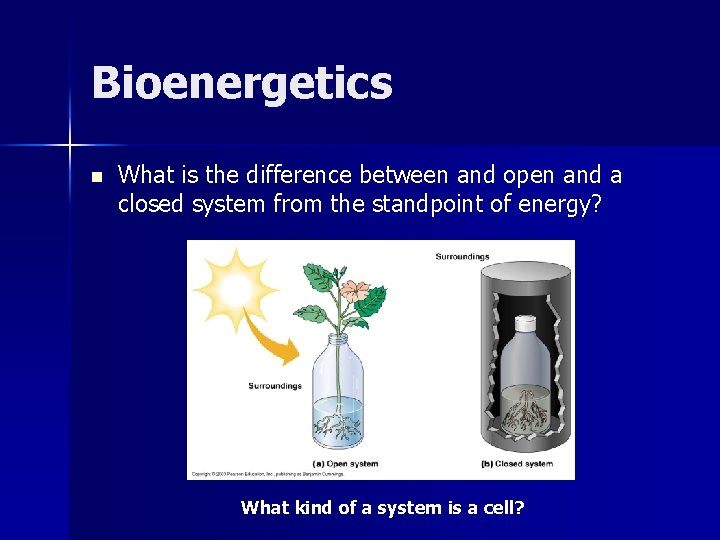
Bioenergetics n What is the difference between and open and a closed system from the standpoint of energy? What kind of a system is a cell?

Bioenergetics n What is the unit used to measure energy changes in cells? n How do we define this unit?

Bioenergetics n What does the first law of thermodynamics say? n What do we mean by the internal energy of a system? energy stored = energy in – energy out

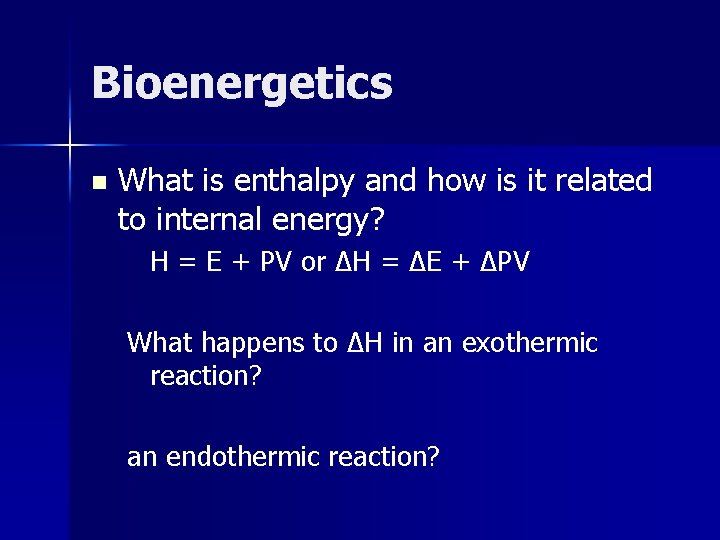
Bioenergetics n What is enthalpy and how is it related to internal energy? H = E + PV or ΔH = ΔE + ΔPV What happens to ΔH in an exothermic reaction? an endothermic reaction?

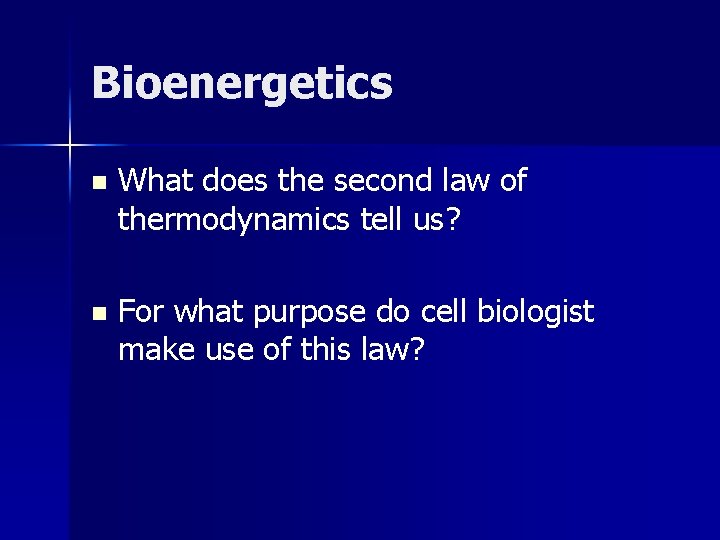
Bioenergetics n What does the second law of thermodynamics tell us? n For what purpose do cell biologist make use of this law?

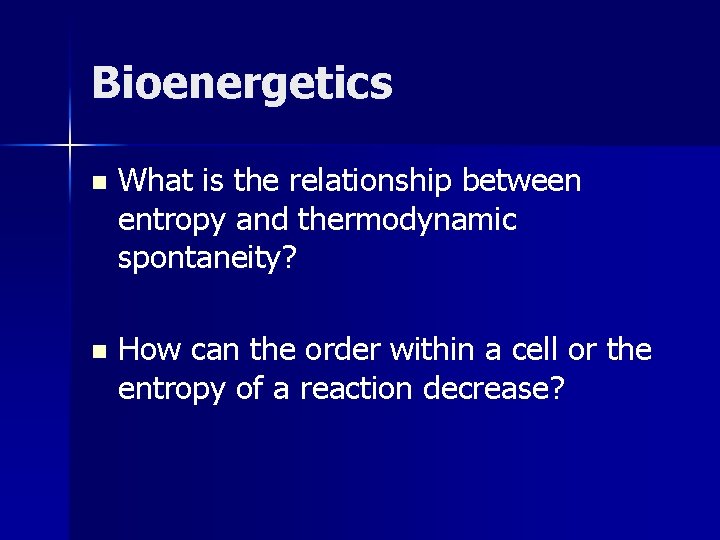
Bioenergetics n What is the relationship between entropy and thermodynamic spontaneity? n How can the order within a cell or the entropy of a reaction decrease?

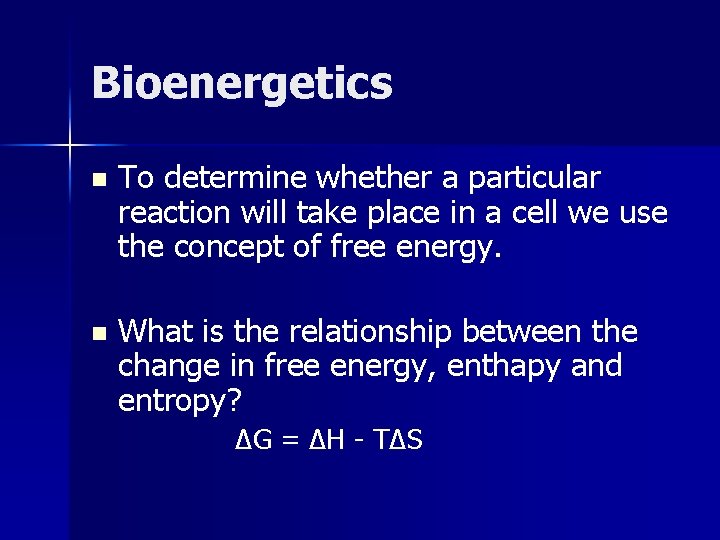
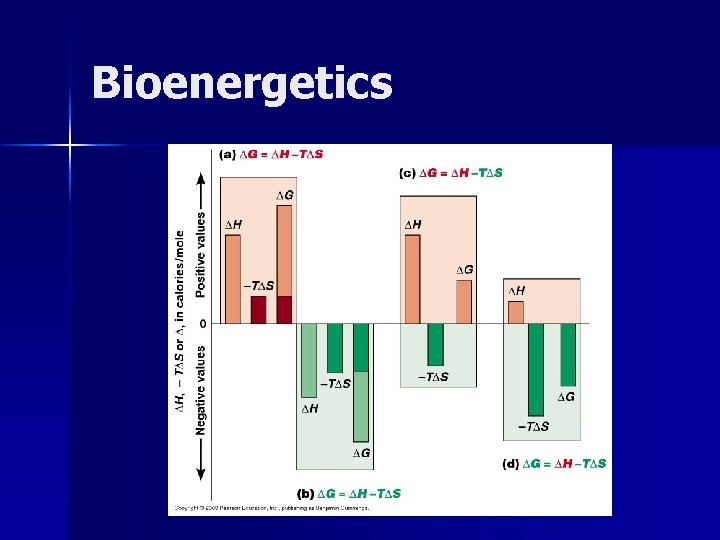
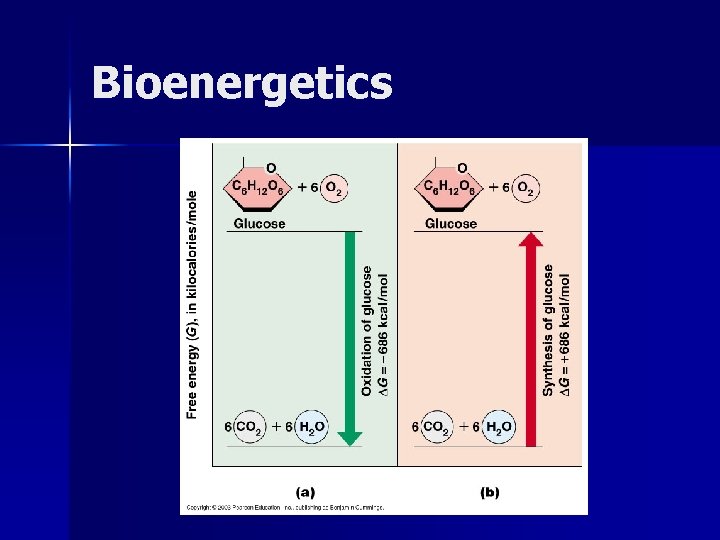
Bioenergetics n To determine whether a particular reaction will take place in a cell we use the concept of free energy. n What is the relationship between the change in free energy, enthapy and entropy? ΔG = ΔH - T ΔS

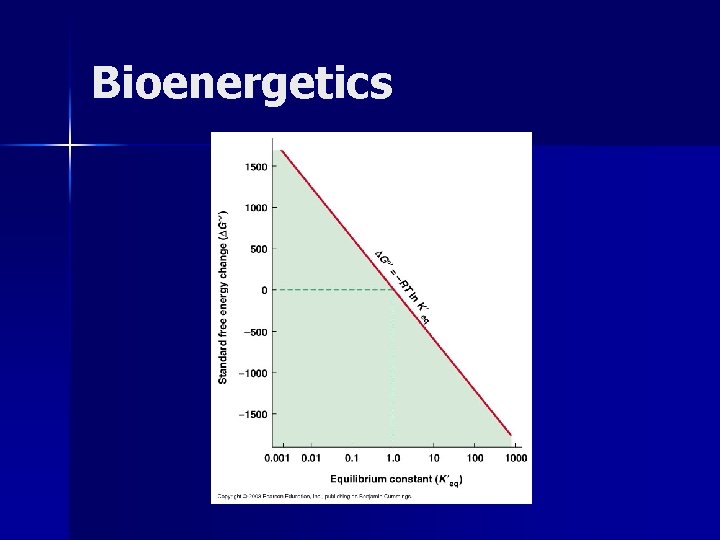
Bioenergetics

Bioenergetics n How can we determine from the value of ΔG whether a reaction will occur spontaneously? n What enables us to characterize reactions as endergonic or exergonic?

Bioenergetics

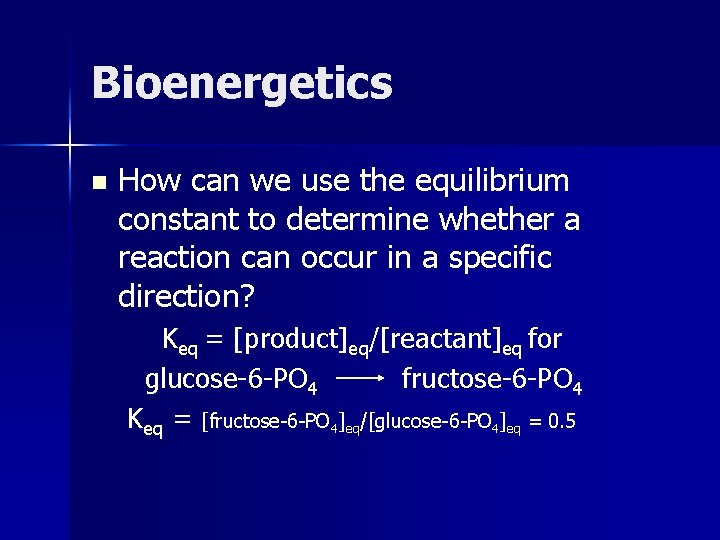
Bioenergetics n How can we use the equilibrium constant to determine whether a reaction can occur in a specific direction? Keq = [product]eq/[reactant]eq for glucose-6 -PO 4 fructose-6 -PO 4 Keq = [fructose-6 -PO 4]eq/[glucose-6 -PO 4]eq = 0. 5

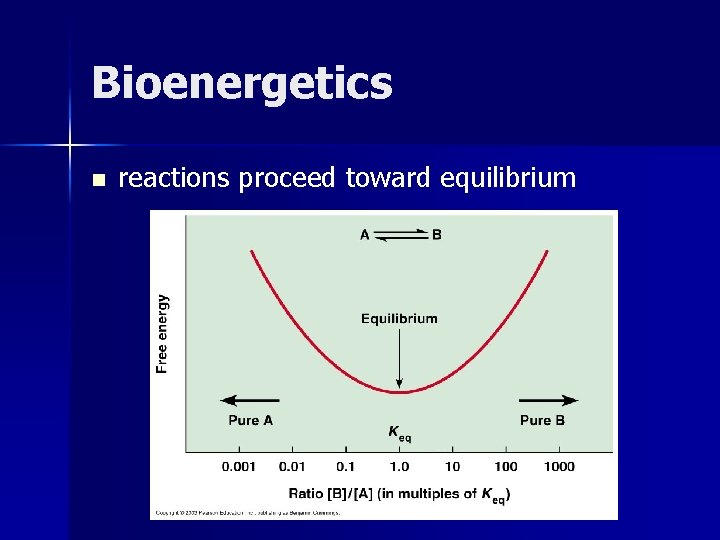
Bioenergetics n reactions proceed toward equilibrium

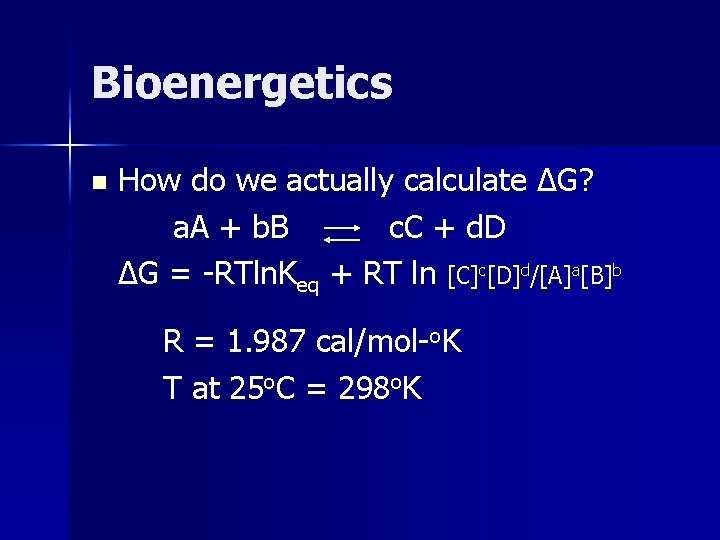
Bioenergetics n How do we actually calculate ΔG? a. A + b. B c. C + d. D ΔG = -RTln. Keq + RT ln [C]c[D]d/[A]a[B]b R = 1. 987 cal/mol-o. K T at 25 o. C = 298 o. K

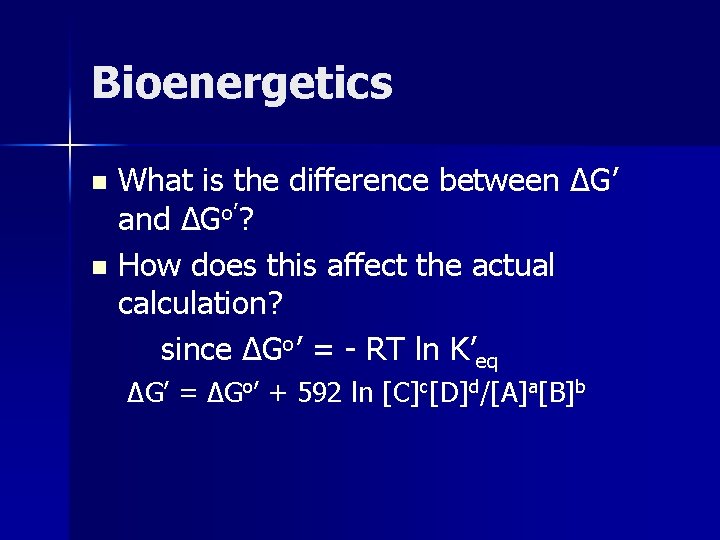
Bioenergetics What is the difference between ΔG’ and ΔGo’? n How does this affect the actual calculation? since ΔGo’ = - RT ln K’eq n ΔG’ = ΔGo’ + 592 ln [C]c[D]d/[A]a[B]b

Bioenergetics

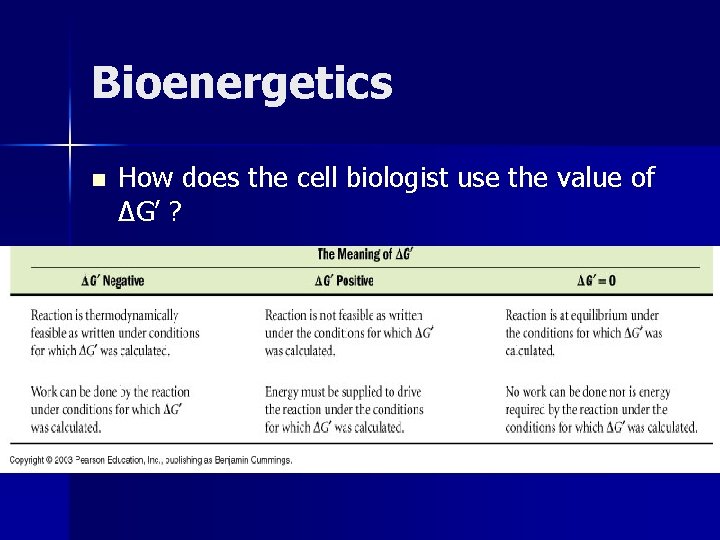
Bioenergetics n How does the cell biologist use the value of ΔG’ ?

Bioenergetics n Why do cellular reactions never reach equilibrium? n What is the steady state and how is it maintained?