Energy Flow in Ecosystems and Biogeochemical Cycles Hierarchy

Energy Flow in Ecosystems and Biogeochemical Cycles

Hierarchy of ecology Individual n Population n Community n Ecosystem n Biosphere n

What’s an Ecosystem? An ecosystem consists of all the organisms (biotic) in a community and the environment (abiotic) with which they interact. n Ecosystems can be as small as the microorganisms living on your skin or as large as the entire biosphere. n
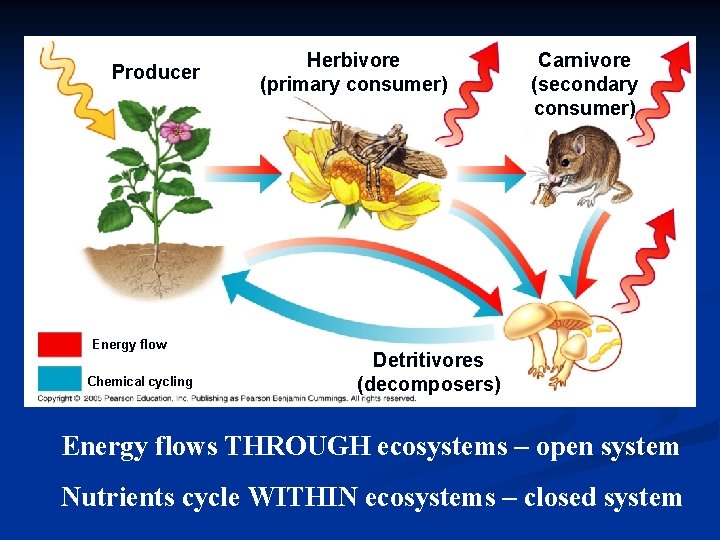
Producer Energy flow Chemical cycling Herbivore (primary consumer) Carnivore (secondary consumer) Detritivores (decomposers) Energy flows THROUGH ecosystems – open system Nutrients cycle WITHIN ecosystems – closed system
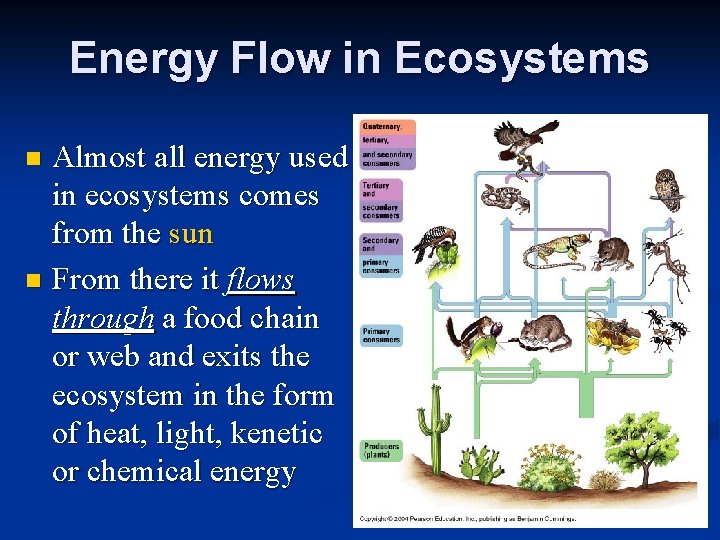
Energy Flow in Ecosystems Almost all energy used in ecosystems comes from the sun n From there it flows through a food chain or web and exits the ecosystem in the form of heat, light, kenetic or chemical energy n
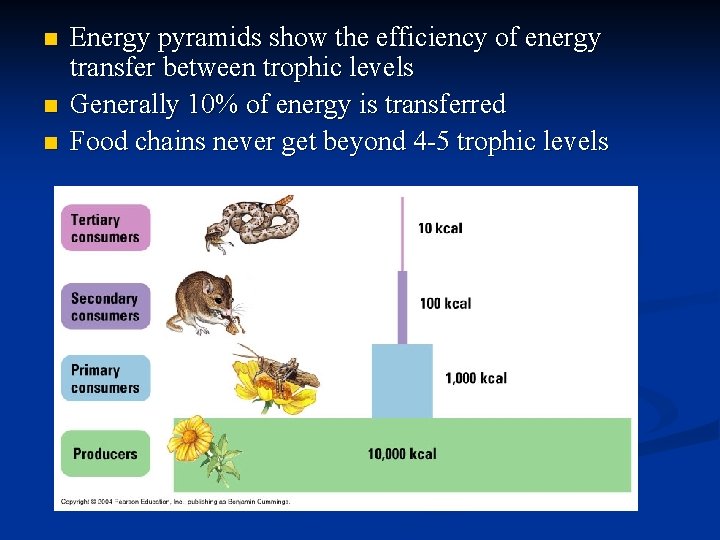
n n n Energy pyramids show the efficiency of energy transfer between trophic levels Generally 10% of energy is transferred Food chains never get beyond 4 -5 trophic levels
Biogeochemical Cycling of Nutrients We have a finite supply of chemical elements n Abiotic reservoir – atmosphere, rocks n Biotic reservoir – within organisms n Biological processing – nitrogen fixation by bacteria, carbon fixation by photosynthesis n Which nutrients/molecules are important?
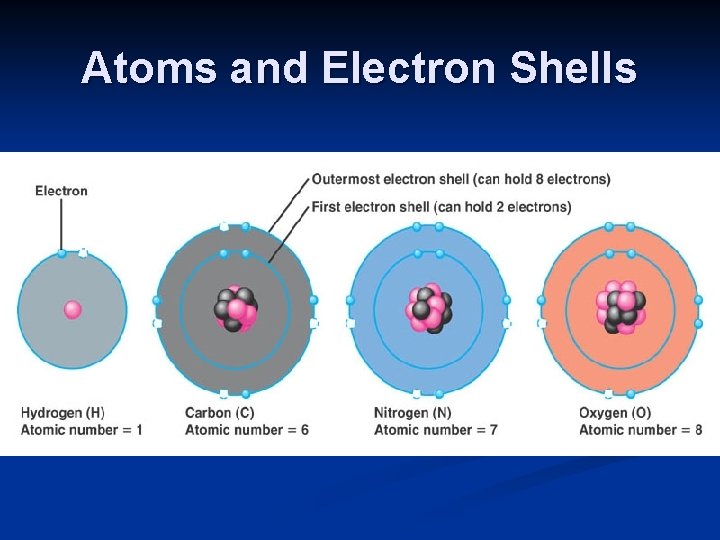
Atoms and Electron Shells
Biogeochemical Cycling of Nutrients n n We have a finite supply of chemical elements Abiotic reservoir – atmosphere, rocks Biotic reservoir – within organisms Biological processing – nitrogen fixation by bacteria, carbon fixation by photosynthesis Water cycle n Carbon cycle n Nitrogen cycle n Phosphorous cycle n } Local OR Global
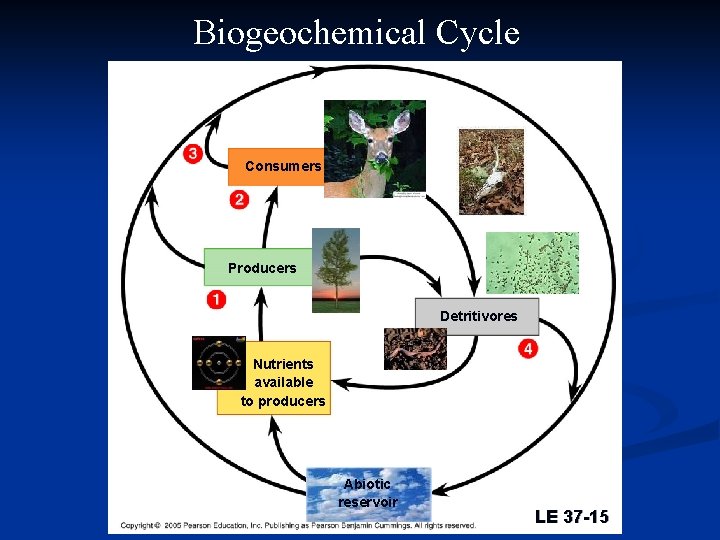
Biogeochemical Cycle Consumers Producers Detritivores Nutrients available to producers Abiotic reservoir LE 37 -15
Biogeochemical Cycling of Nutrients n n We have a finite supply of chemical elements Abiotic reservoir – atmosphere, rocks Biotic reservoir – within organisms Lookprocessing on page 1232 andfixation learn by Biological – nitrogen bacteria, the carbon cycles fixation by photosynthesis Water cycle n Carbon cycle n Nitrogen cycle n Phosphorous cycle n } Local OR Global

What’s so great about water? Polar molecule n High heat capacity – doesn’t change temperature rapidly n Evaporative cooling n Cohesion and adhesion n “Universal” solvent n Lower density when solid – ice floats n Organisms are made of water n

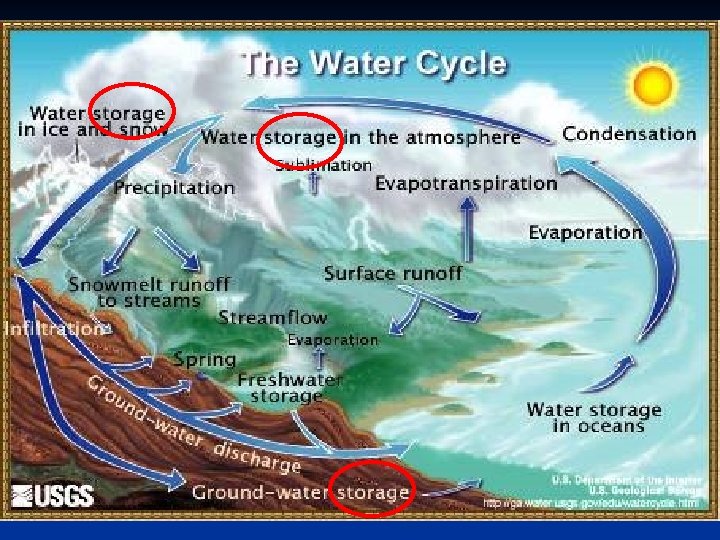
Water Cycle n n n Solar energy drives the global water cycle – Precipitation – Evaporation – Transpiration Water cycles between the land, oceans, and atmosphere Forest destruction and irrigation affect the water cycle
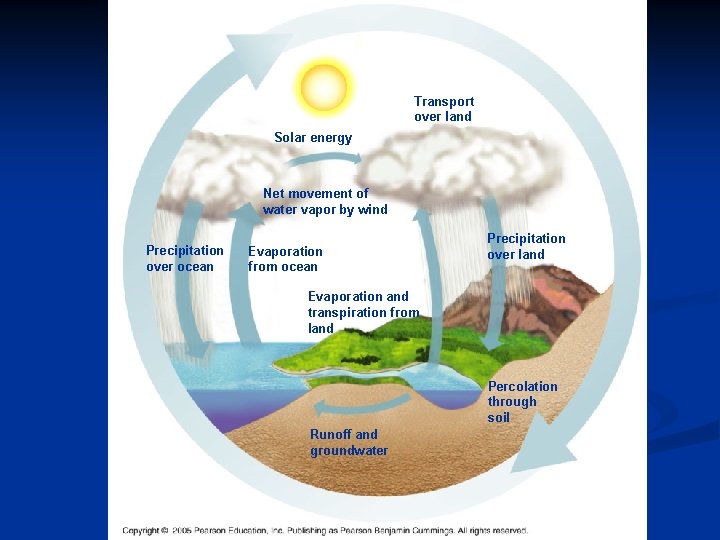
Transport over land Solar energy Net movement of water vapor by wind Precipitation over ocean Evaporation from ocean Precipitation over land Evaporation and transpiration from land Percolation through soil Runoff and groundwater
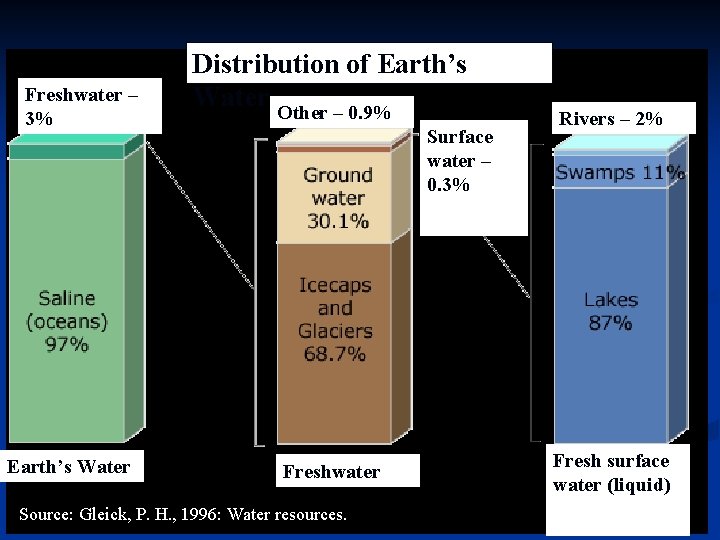
Freshwater – 3% Earth’s Water Distribution of Earth’s Water Other – 0. 9% Surface water – 0. 3% Freshwater Source: Gleick, P. H. , 1996: Water resources. Rivers – 2% Fresh surface water (liquid)
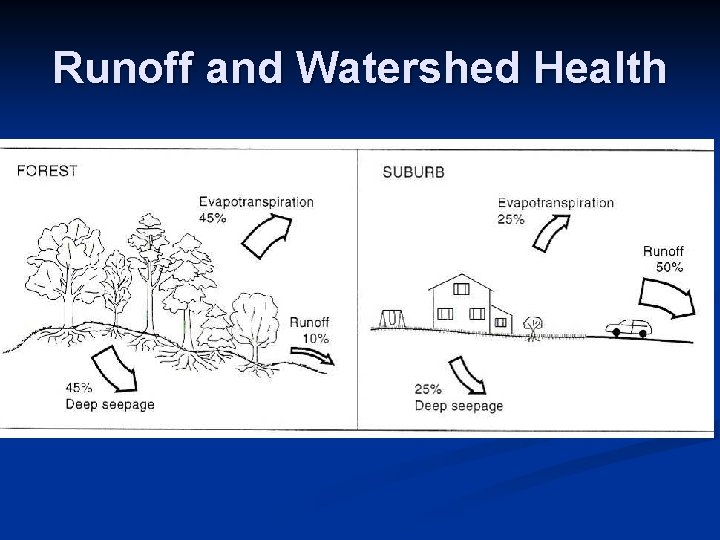
Runoff and Watershed Health

WATER A combination of factors threaten freshwater ecosystems • Acid precipitation • Climate warming • Changes in land use
Biogeochemical Cycling of Nutrients n n We have a finite supply of chemical elements Abiotic reservoir – atmosphere, rocks Biotic reservoir – within organisms Biological processing – nitrogen fixation by bacteria, carbon fixation by photosynthesis Water cycle n Carbon cycle n Nitrogen cycle n Phosphorous cycle n } Local OR Global

Carbon Cycle • Abiotic reservoirs = atmosphere, sedimentary rocks, dissolved carbon in oceans, and fossil fuels – Taken from the atmosphere by photosynthesis – Used to make organic molecules – Decomposed by detritivores – Returned to the atmosphere by cellular respiration
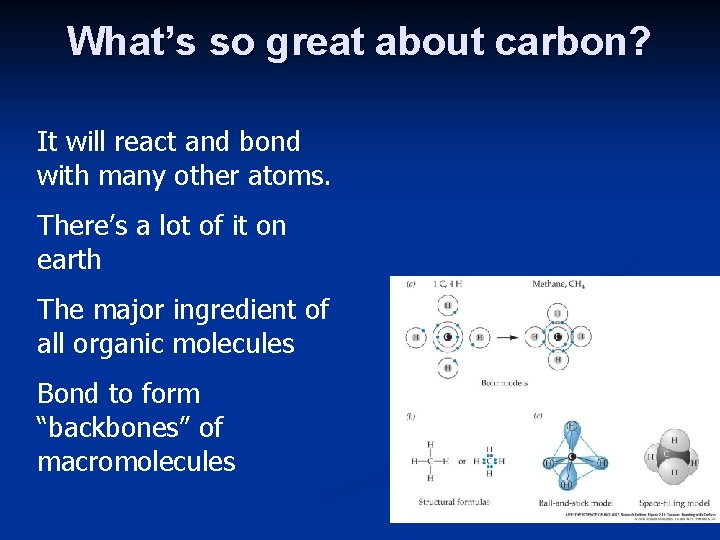
What’s so great about carbon? It will react and bond with many other atoms. There’s a lot of it on earth The major ingredient of all organic molecules Bond to form “backbones” of macromolecules
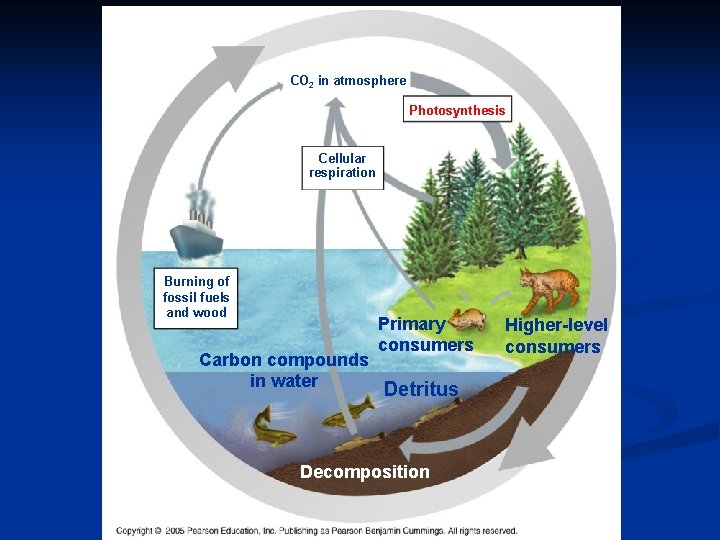
CO 2 in atmosphere Photosynthesis Cellular respiration Burning of fossil fuels and wood Primary consumers Carbon compounds in water Detritus Decomposition Higher-level consumers

CONNECTION – Global Warming n Burning of fossil fuels and wood is increasing the amount of CO 2 and other greenhouse gases in the air n Correlation with increased global temperature n The greenhouse effect n Natural phenomenon is essential for life on Earth n Rapidly increasing CO 2 is making global warming a danger
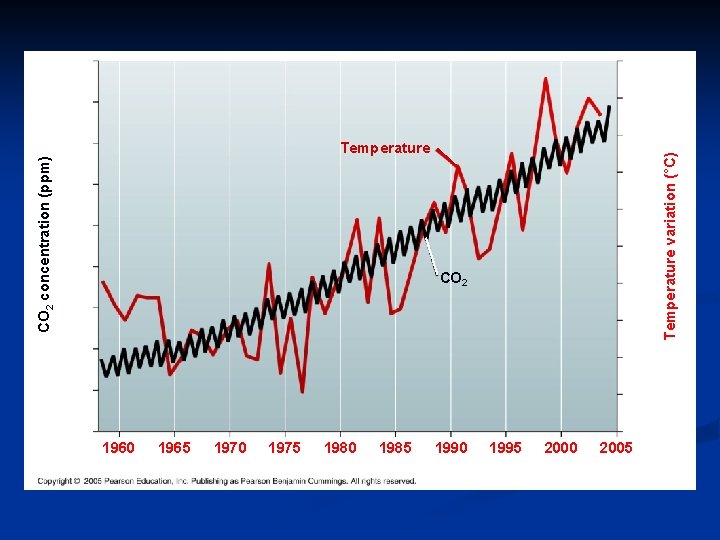
390 1. 05 380 0. 90 0. 75 Temperature 0. 60 360 0. 45 350 0. 30 340 CO 2 0. 15 330 0 320 Temperature variation (°C) CO 2 concentration (ppm) 370 – 0. 15 310 – 0. 30 300 – 0. 45 2005 1960 1965 1970 1975 1980 1985 Year 1990 1995 2000
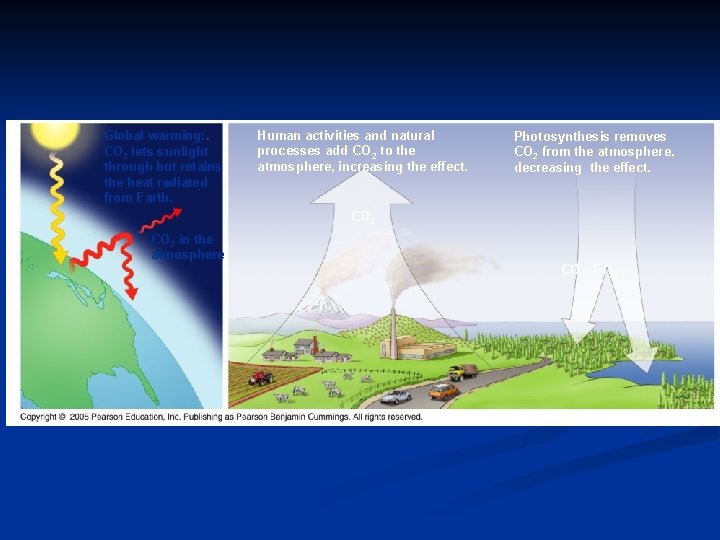
Global warming: CO 2 lets sunlight through but retains the heat radiated from Earth. Human activities and natural processes add CO 2 to the atmosphere, increasing the effect. Photosynthesis removes CO 2 from the atmosphere, decreasing the effect. CO 2 in the atmosphere CO 2
Biogeochemical Cycling of Nutrients n n We have a finite supply of chemical elements Abiotic reservoir – atmosphere, rocks Biotic reservoir – within organisms Biological processing – nitrogen fixation by bacteria, carbon fixation by photosynthesis Water cycle n Carbon cycle n Nitrogen cycle n Phosphorous cycle n } Local OR Global
What’s so great about nitrogen? Ingredient of proteins and nucleic acids n Essential to the functioning and structure of all organisms n Crucial for plants, but limited in quantity n
Nitrogen Cycle The nitrogen cycle relies heavily on bacteria • Atmospheric N 2 is not available to plants – Soil bacteria convert gaseous N 2 to usable ammonium (NH 4+) and nitrate (NO 3 -) – Some NH 4+ and NO 3 - are made by chemical reactions in the atmosphere
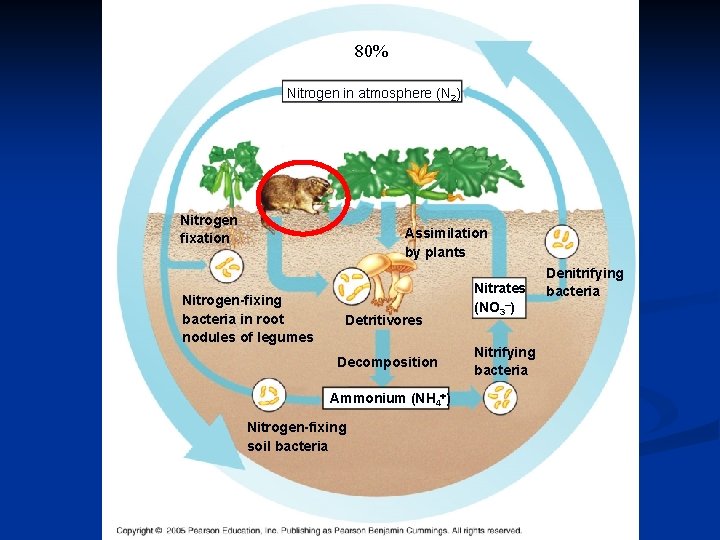
80% Nitrogen in atmosphere (N 2) Nitrogen fixation Assimilation by plants Nitrogen-fixing bacteria in root nodules of legumes Detritivores Decomposition Ammonium (NH 4 ) Nitrogen-fixing soil bacteria Nitrates (NO 3–) Nitrifying bacteria Denitrifying bacteria
Biogeochemical Cycling of Nutrients n n We have a finite supply of chemical elements Abiotic reservoir – atmosphere, rocks Biotic reservoir – within organisms Biological processing – nitrogen fixation by bacteria, carbon fixation by photosynthesis Water cycle n Carbon cycle n Nitrogen cycle n Phosphorous cycle n } Local OR Global
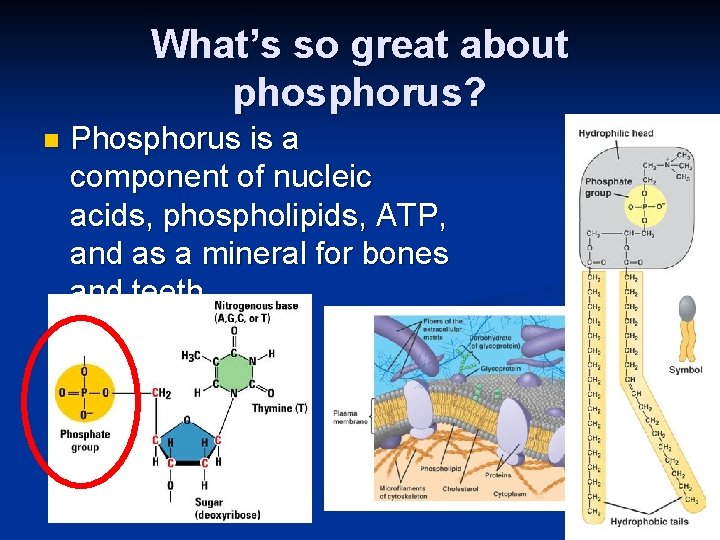
What’s so great about phosphorus? n Phosphorus is a component of nucleic acids, phospholipids, ATP, and as a mineral for bones and teeth.

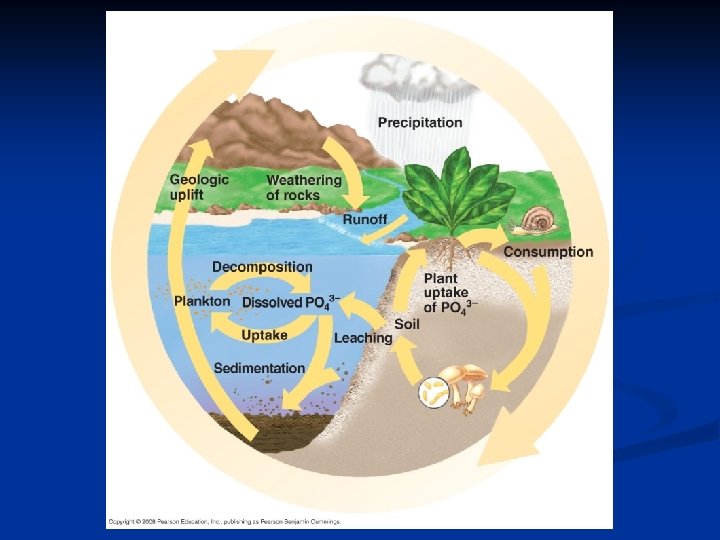
Phosphorus Cycle Depends on the weathering of rock • Phosphorus and other soil minerals are recycled locally • Weathering of rock adds PO 43 - to soil – Slow process makes amount of phosphorus available to plants low
Runoff Sedimentation

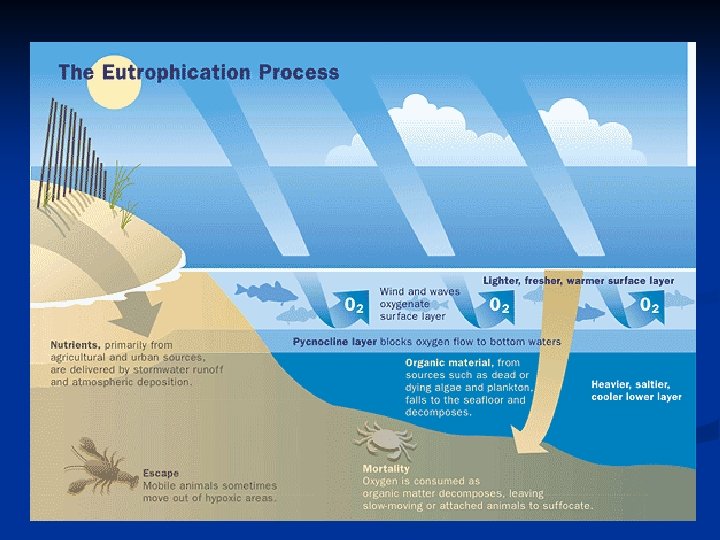
Connections • • Nutrient runoff from agricultural lands and large livestock operations may cause excessive algal growth This cultural eutrophication reduces species diversity and harms water quality
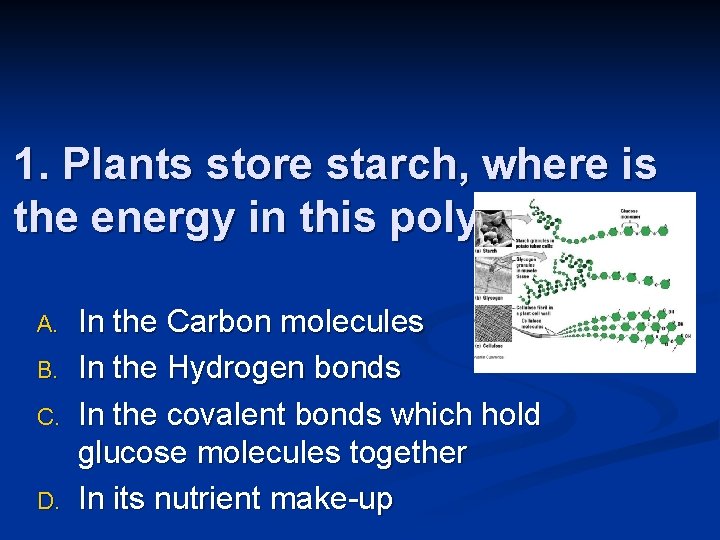
1. Plants store starch, where is the energy in this polymer? A. B. C. D. In the Carbon molecules In the Hydrogen bonds In the covalent bonds which hold glucose molecules together In its nutrient make-up

3. A Covalent bond is formed when atoms SHARE electrons. TRUE FALSE

5. Fill in the blanks: Energy flows_____ ecosystems, while nutrients cycle ______ ecosystems. A. B. Within, Through, Within
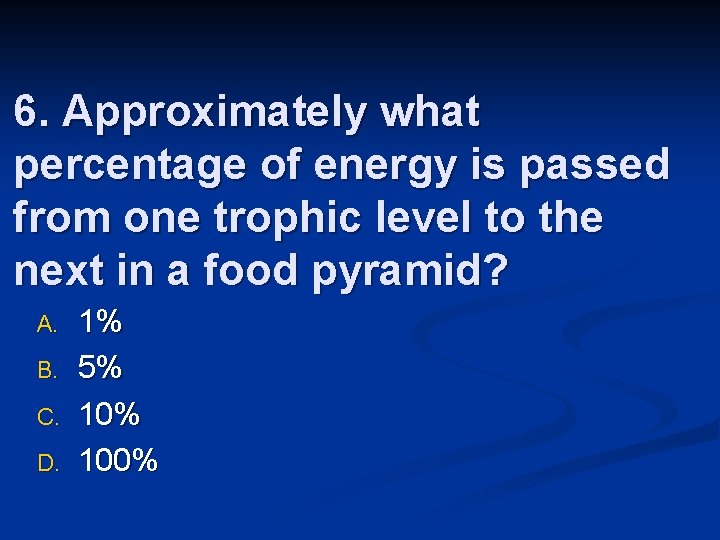
6. Approximately what percentage of energy is passed from one trophic level to the next in a food pyramid? A. B. C. D. 1% 5% 100%

7. How is Carbon released back to the Atmosphere? A. B. C. D. E. Through respiration Through photosynthesis By burning fossil fuels All of the above Both A and C
- Slides: 40