ENERGY FLOW and CELLULAR RESPIRATION LECTURE 7 Pages






- Slides: 31

ENERGY FLOW and CELLULAR RESPIRATION LECTURE 7 Pages 107, 110, 112 -113 (Chapter 6) Pages 133 -139 (Chapter 8)

HOMEWORK- Week 7 Read pages 112 -113, Chapter 6 Read pages 133 -149, Chapter 8 Do all of the chapter 8 self-tests

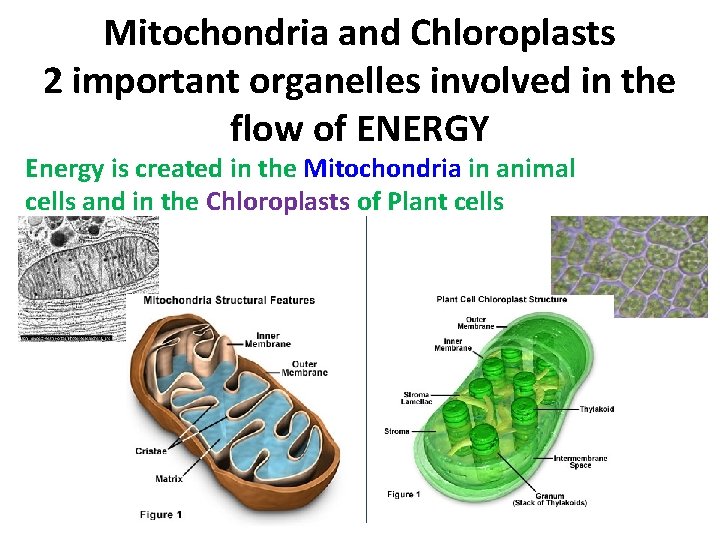
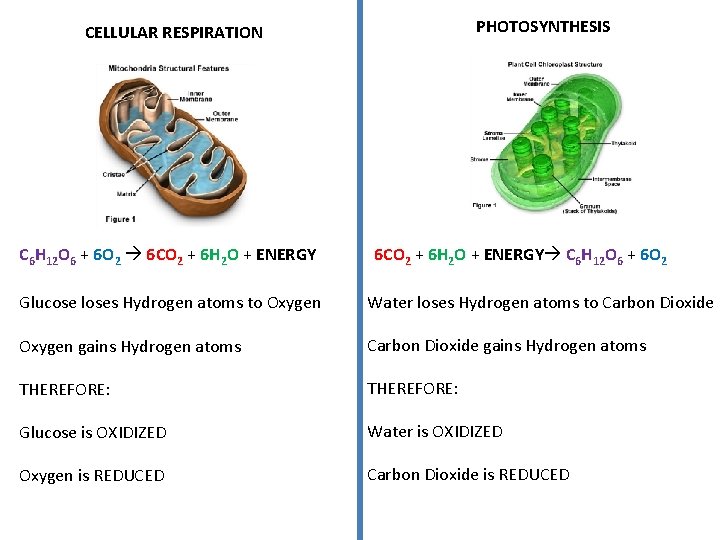
Mitochondria and Chloroplasts 2 important organelles involved in the flow of ENERGY Energy is created in the Mitochondria in animal cells and in the Chloroplasts of Plant cells

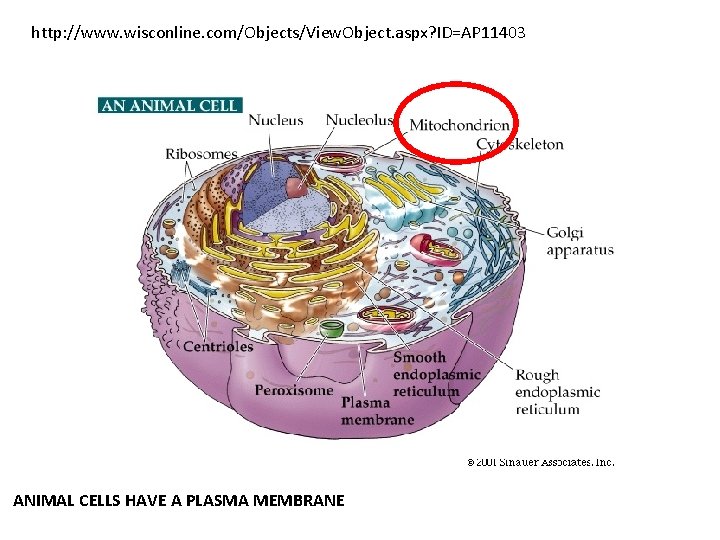
http: //www. wisconline. com/Objects/View. Object. aspx? ID=AP 11403 ANIMAL CELLS HAVE A PLASMA MEMBRANE

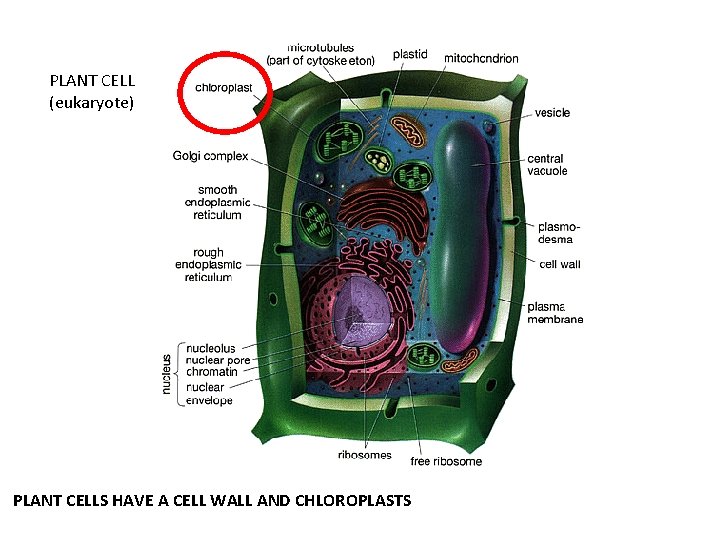
PLANT CELL (eukaryote) PLANT CELLS HAVE A CELL WALL AND CHLOROPLASTS

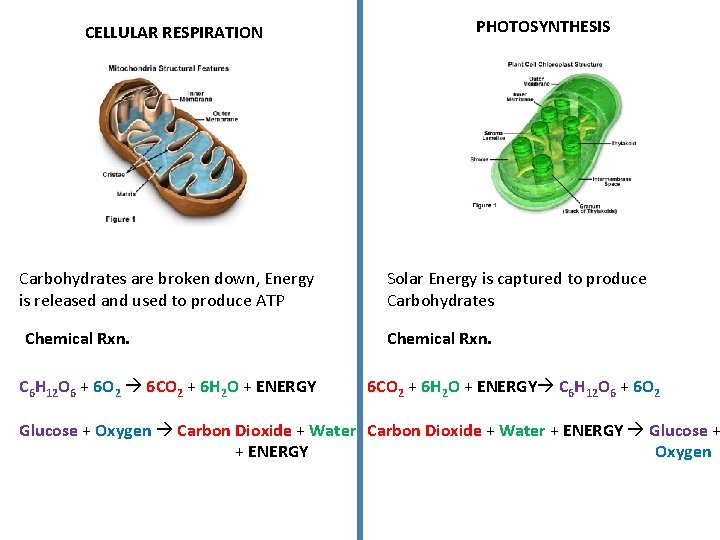
CELLULAR RESPIRATION Carbohydrates are broken down, Energy is released and used to produce ATP Chemical Rxn. C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O + ENERGY PHOTOSYNTHESIS Solar Energy is captured to produce Carbohydrates Chemical Rxn. 6 CO 2 + 6 H 2 O + ENERGY C 6 H 12 O 6 + 6 O 2 Glucose + Oxygen Carbon Dioxide + Water + ENERGY Glucose + + ENERGY Oxygen

PHOTOSYNTHESIS CELLULAR RESPIRATION C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O + ENERGY C 6 H 12 O 6 + 6 O 2 Glucose loses Hydrogen atoms to Oxygen Water loses Hydrogen atoms to Carbon Dioxide Oxygen gains Hydrogen atoms Carbon Dioxide gains Hydrogen atoms THEREFORE: Glucose is OXIDIZED Water is OXIDIZED Oxygen is REDUCED Carbon Dioxide is REDUCED

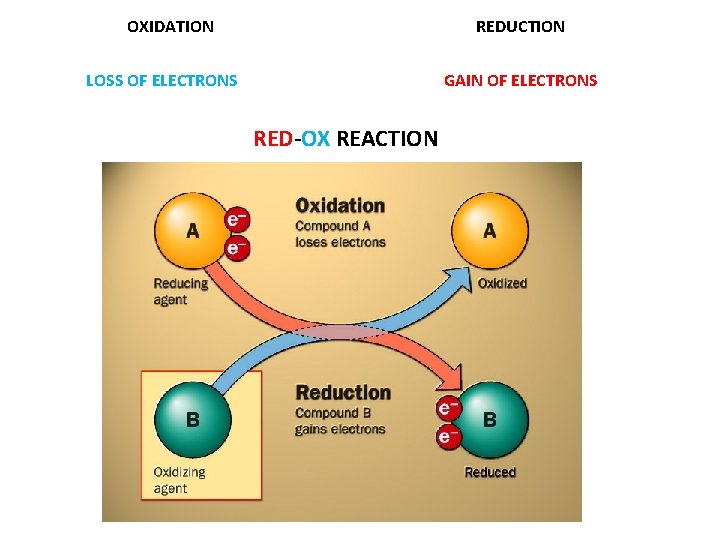
OXIDATION REDUCTION LOSS OF ELECTRONS GAIN OF ELECTRONS RED-OX REACTION

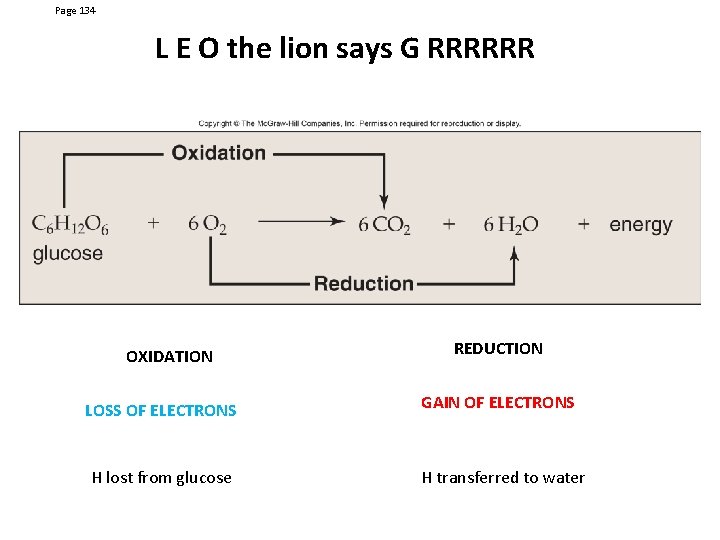
Page 134 L E O the lion says G RRRRRR OXIDATION REDUCTION LOSS OF ELECTRONS GAIN OF ELECTRONS H lost from glucose H transferred to water

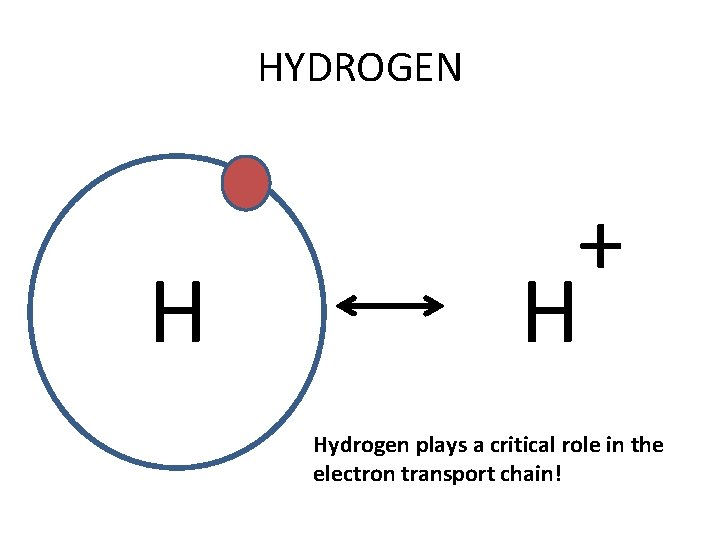
HYDROGEN H + H Hydrogen plays a critical role in the electron transport chain!

REMEMBER!! CELLULAR RESPIRATION and PHOTOSYNTHESIS are chemical processes by which the flow of ENERGY through various molecules (ex. Proteins) occurs to produce either: 1. CO 2, Water and ENERGY (cellular respiration) 2. Glucose and Oxygen (photosynthesis) THE FLOW OF ENERGY REFERS TO THE TRANSFER OF ELECTRONS and the MOVEMENT OF HYDROGEN IONS FROM ONE MOLECULE TO ANOTHER

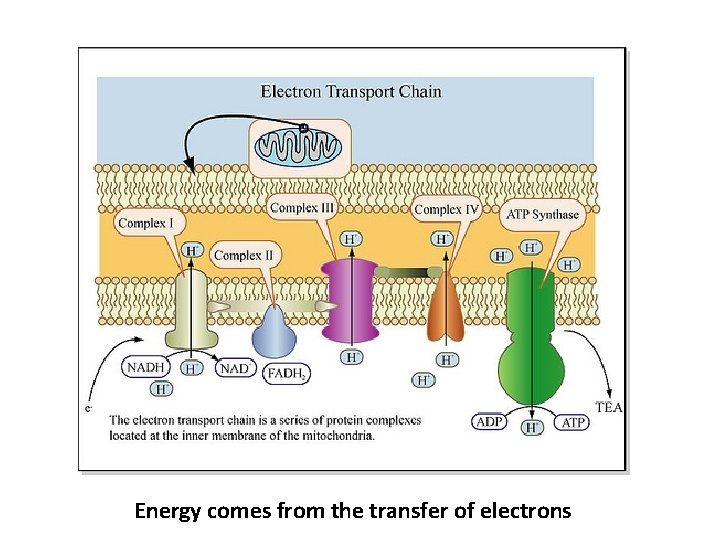
Energy comes from the transfer of electrons

CELLULAR RESPIRATION

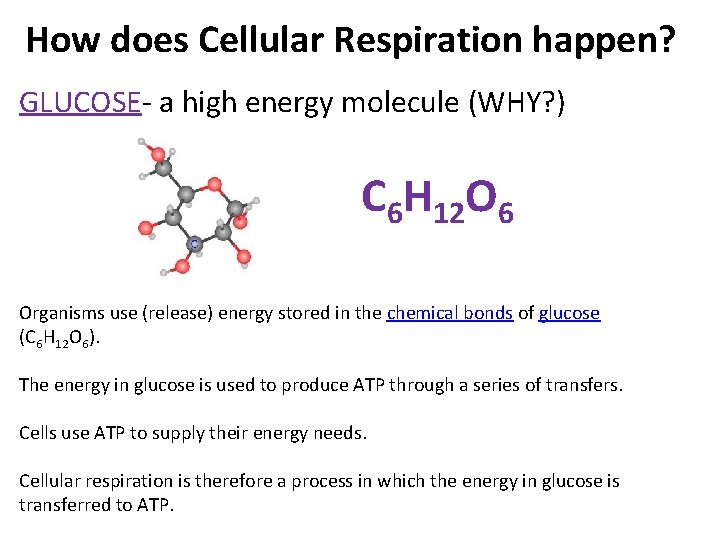
How does Cellular Respiration happen? GLUCOSE- a high energy molecule (WHY? ) C 6 H 12 O 6 Organisms use (release) energy stored in the chemical bonds of glucose (C 6 H 12 O 6). The energy in glucose is used to produce ATP through a series of transfers. Cells use ATP to supply their energy needs. Cellular respiration is therefore a process in which the energy in glucose is transferred to ATP.

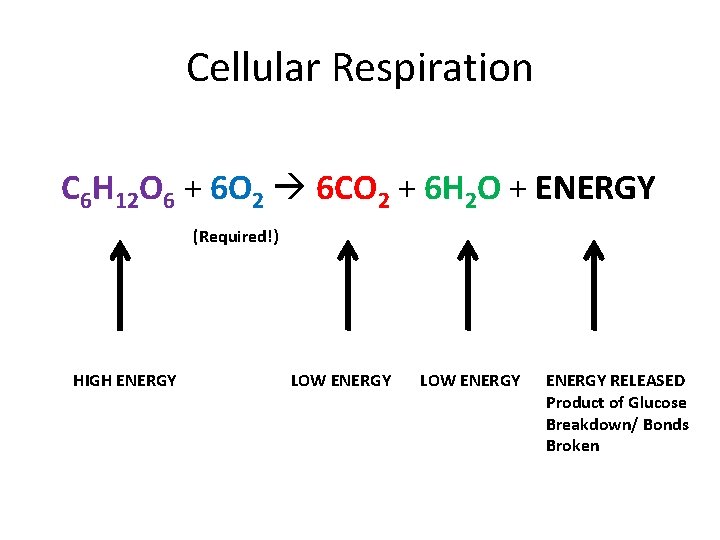
Cellular Respiration C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O + ENERGY (Required!) HIGH ENERGY LOW ENERGY RELEASED Product of Glucose Breakdown/ Bonds Broken

GLUCOSE BREAKDOWN • In Cellular Respiration, glucose is NOT broken down all at once. WHY? Because too much energy would be lost in the form of unusable heat! Cell respiration occurs in a series of steps or metabolic reactions to retain as much energy as possible

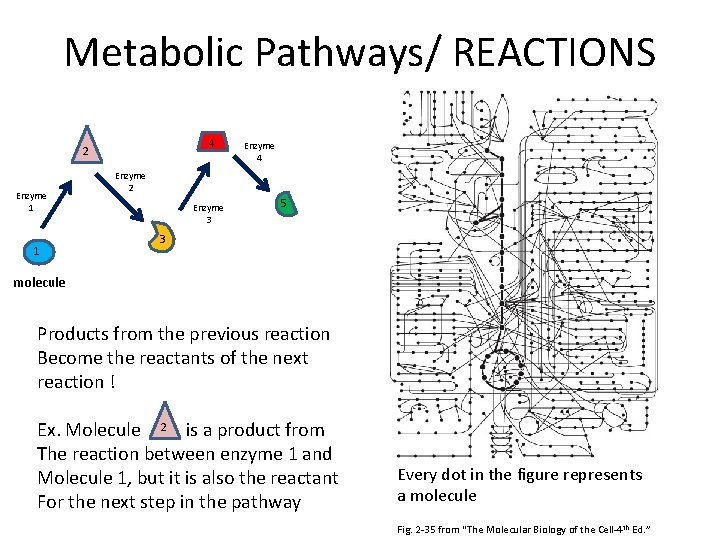
Metabolic Pathways/ REACTIONS 4 2 Enzyme 1 1 Enzyme 4 Enzyme 2 Enzyme 3 5 3 molecule Products from the previous reaction Become the reactants of the next reaction ! Ex. Molecule 2 is a product from The reaction between enzyme 1 and Molecule 1, but it is also the reactant For the next step in the pathway Every dot in the figure represents a molecule Fig. 2 -35 from “The Molecular Biology of the Cell-4 th Ed. ”

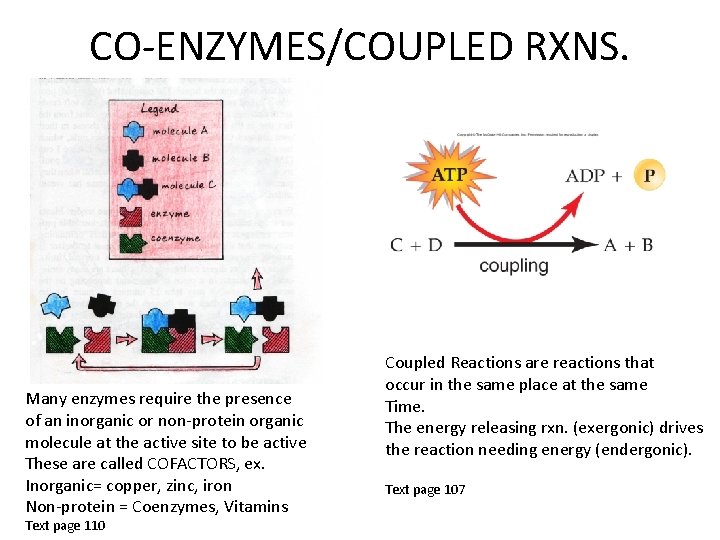
CO-ENZYMES/COUPLED RXNS. Many enzymes require the presence of an inorganic or non-protein organic molecule at the active site to be active These are called COFACTORS, ex. Inorganic= copper, zinc, iron Non-protein = Coenzymes, Vitamins Text page 110 Coupled Reactions are reactions that occur in the same place at the same Time. The energy releasing rxn. (exergonic) drives the reaction needing energy (endergonic). Text page 107

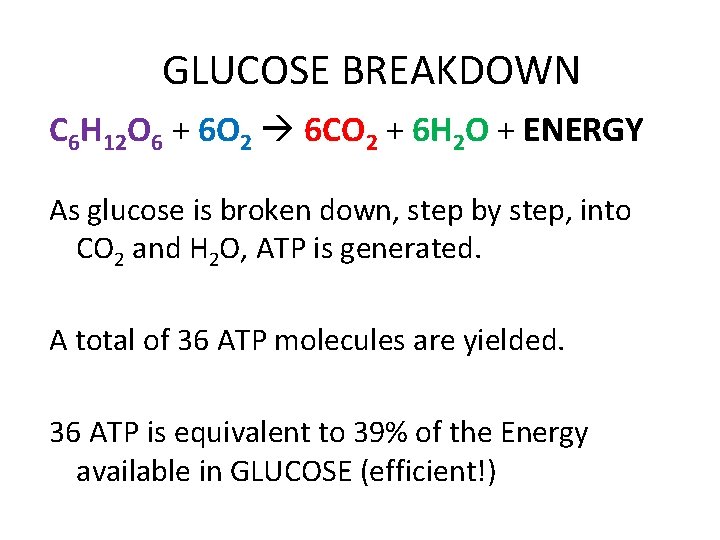
GLUCOSE BREAKDOWN C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O + ENERGY As glucose is broken down, step by step, into CO 2 and H 2 O, ATP is generated. A total of 36 ATP molecules are yielded. 36 ATP is equivalent to 39% of the Energy available in GLUCOSE (efficient!)

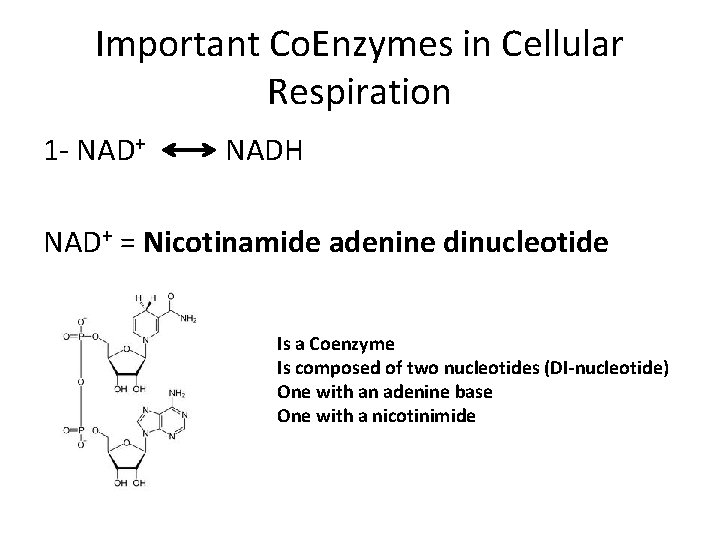
Important Co. Enzymes in Cellular Respiration 1 - NAD+ NADH NAD+ = Nicotinamide adenine dinucleotide Is a Coenzyme Is composed of two nucleotides (DI-nucleotide) One with an adenine base One with a nicotinimide

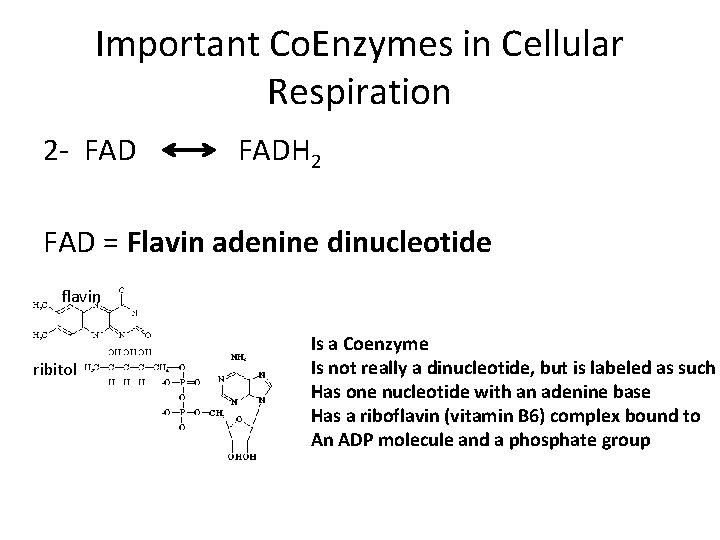
Important Co. Enzymes in Cellular Respiration 2 - FADH 2 FAD = Flavin adenine dinucleotide flavin ribitol Is a Coenzyme Is not really a dinucleotide, but is labeled as such Has one nucleotide with an adenine base Has a riboflavin (vitamin B 6) complex bound to An ADP molecule and a phosphate group

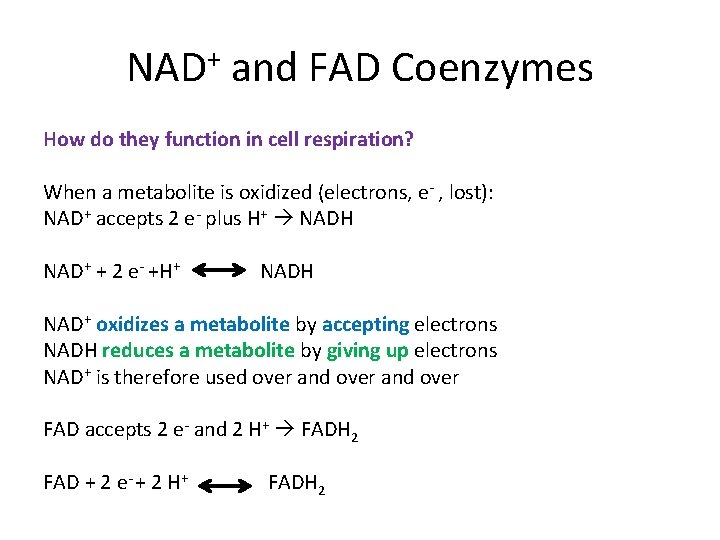
NAD+ and FAD Coenzymes How do they function in cell respiration? When a metabolite is oxidized (electrons, e- , lost): NAD+ accepts 2 e- plus H+ NADH NAD+ + 2 e- +H+ NADH NAD+ oxidizes a metabolite by accepting electrons NADH reduces a metabolite by giving up electrons NAD+ is therefore used over and over FAD accepts 2 e- and 2 H+ FADH 2 FAD + 2 e- + 2 H+ FADH 2

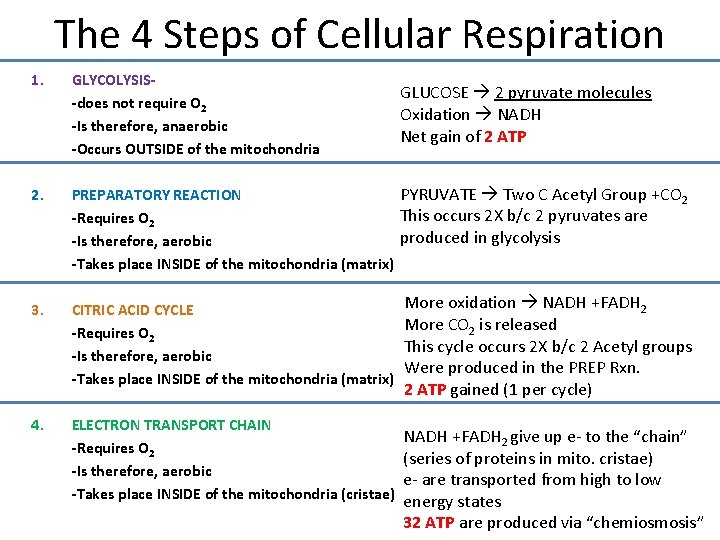
The 4 Steps of Cellular Respiration 1. GLYCOLYSIS-does not require O 2 -Is therefore, anaerobic -Occurs OUTSIDE of the mitochondria 2. PYRUVATE Two C Acetyl Group +CO 2 PREPARATORY REACTION This occurs 2 X b/c 2 pyruvates are -Requires O 2 produced in glycolysis -Is therefore, aerobic -Takes place INSIDE of the mitochondria (matrix) 3. CITRIC ACID CYCLE -Requires O 2 -Is therefore, aerobic -Takes place INSIDE of the mitochondria (matrix) 4. ELECTRON TRANSPORT CHAIN -Requires O 2 -Is therefore, aerobic -Takes place INSIDE of the mitochondria (cristae) GLUCOSE 2 pyruvate molecules Oxidation NADH Net gain of 2 ATP More oxidation NADH +FADH 2 More CO 2 is released This cycle occurs 2 X b/c 2 Acetyl groups Were produced in the PREP Rxn. 2 ATP gained (1 per cycle) NADH +FADH 2 give up e- to the “chain” (series of proteins in mito. cristae) e- are transported from high to low energy states 32 ATP are produced via “chemiosmosis”

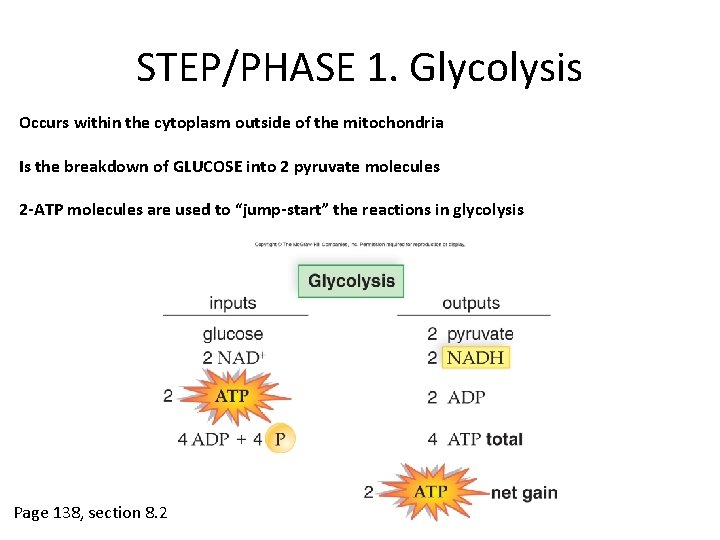
STEP/PHASE 1. Glycolysis Occurs within the cytoplasm outside of the mitochondria Is the breakdown of GLUCOSE into 2 pyruvate molecules 2 -ATP molecules are used to “jump-start” the reactions in glycolysis Page 138, section 8. 2

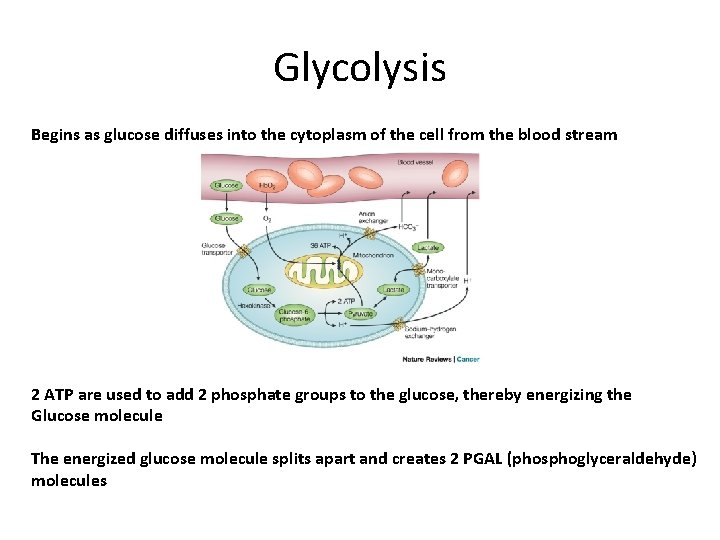
Glycolysis Begins as glucose diffuses into the cytoplasm of the cell from the blood stream 2 ATP are used to add 2 phosphate groups to the glucose, thereby energizing the Glucose molecule The energized glucose molecule splits apart and creates 2 PGAL (phosphoglyceraldehyde) molecules

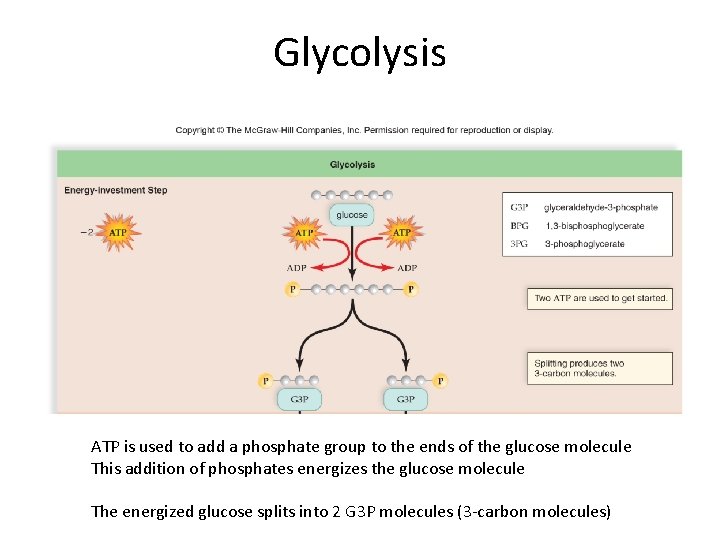
Glycolysis ATP is used to add a phosphate group to the ends of the glucose molecule This addition of phosphates energizes the glucose molecule The energized glucose splits into 2 G 3 P molecules (3 -carbon molecules)

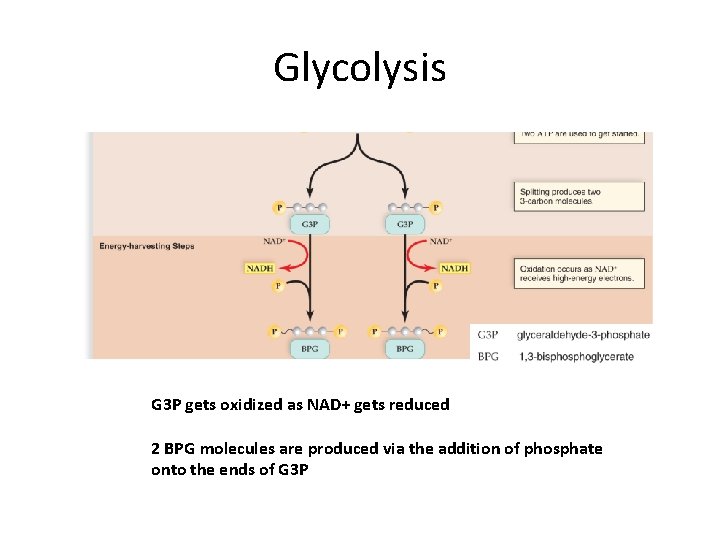
Glycolysis G 3 P gets oxidized as NAD+ gets reduced 2 BPG molecules are produced via the addition of phosphate onto the ends of G 3 P

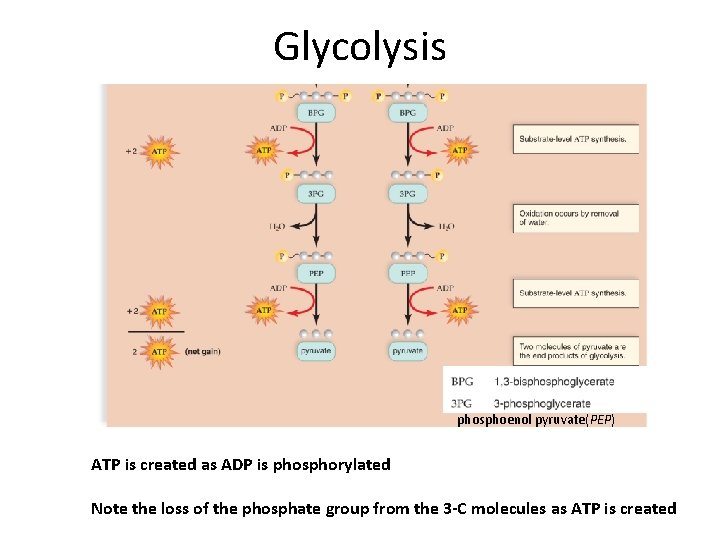
Glycolysis phosphoenol pyruvate(PEP) ATP is created as ADP is phosphorylated Note the loss of the phosphate group from the 3 -C molecules as ATP is created

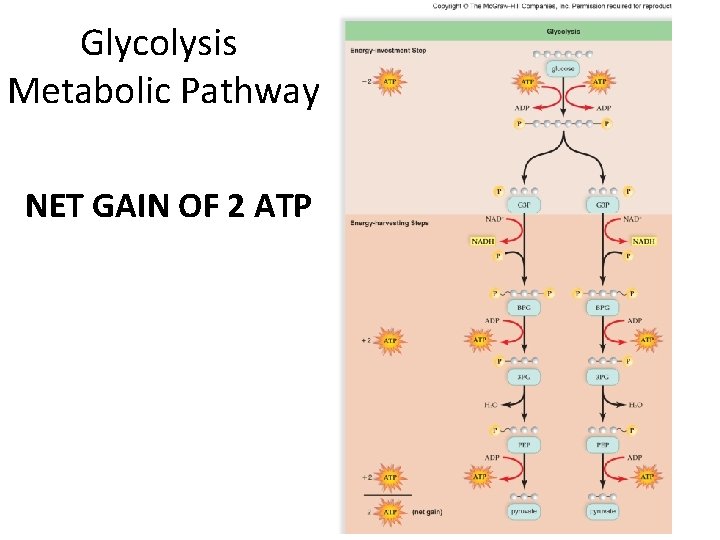
Glycolysis Metabolic Pathway NET GAIN OF 2 ATP

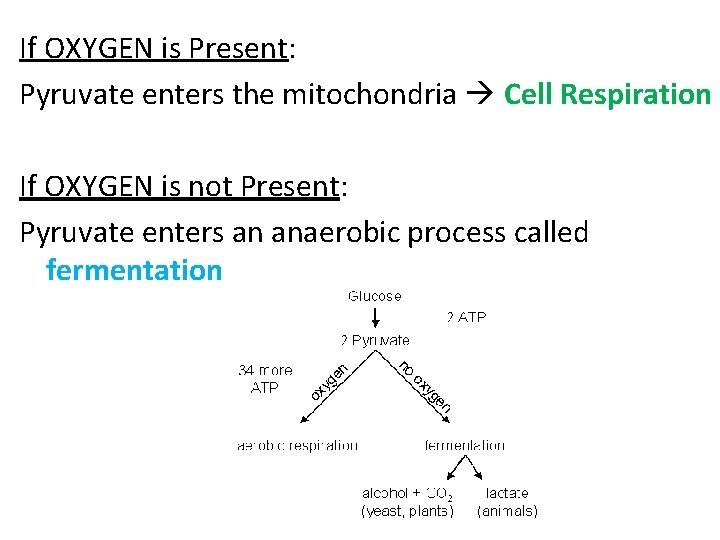
If OXYGEN is Present: Pyruvate enters the mitochondria Cell Respiration If OXYGEN is not Present: Pyruvate enters an anaerobic process called fermentation

QUESTIONS? ?