Energy Enzymes Metabolism Chapter 6 Energy Different forms

Energy, Enzymes, & Metabolism Chapter 6
Energy ¢ Different forms Chemical l Electric l Heat l Light l Mechanical l ¢ Potential Energy – stored energy l ¢ Stored in covalent bonds Kinetic energy – energy of movement
Metabolism ¢ Sum total of chemical reactions that are occurring continuously l Anabolic Reaction (anabolism) • Link simple molecules to form more complex (making protein) l Catabolic Reaction (catabolism) • Break down complex molecules into simpler ones; releasing the energy
Laws of Thermodynamics Thermo, “energy”; dynamics, “change” ¢ 1 st law – Energy is neither created nor destroyed ¢ l ¢ Total energy before & after the conversion is the same 2 nd law – Disorder tends to increase l When energy is converted, some energy becomes unavailable to do work

Transforming Energy Light energy hits solar cell & converted to electric energy ¢ Stored as chemical energy in a battery ¢ At night the chemical energy is converted to electric energy that is converted to light energy ¢

Transforming Energy ¢ ¢ Light energy absorbed by photosynthetic pigments in soybean plants Use this energy to create concentrated chemical energy Soybeans harvested & their oil makes candles Candle burns, the stored energy is transformed into light energy & thermal energy

2 nd law of thermodynamics ¢ Total energy = usable energy + unusable energy Total energy- enthalpy (H) l Usable energy- free energy (G) l Unusable energy- entropy (S) l • Measure of the disorder of the system multipled by the absolute temperature (T) H = G + TS


Measurement of energy ¢ Change in energy can be measured in calories or joules. l Calorie = amount of heat energy needed to raise the temperature of 1 gram water from 14. 5 to 15. 5 C. l Joules = energy measure in the SI system • 1 J = 0. 239 cal • 1 cal = 4. 184 J

Food Calories Energy in food is Calories ¢ One Calorie (food calorie) = 1000 calories or 1 kcal ¢ l Candy bar contains 230 Cal • If burned it would produce 230, 000 cal or 962 k. J of thermal energy ¢ 1 g of glucose will release 17 k. J

Energy in Food ¢ For each group, rank the foods by the amount of energy you think they contain (1 -least, 4 most)
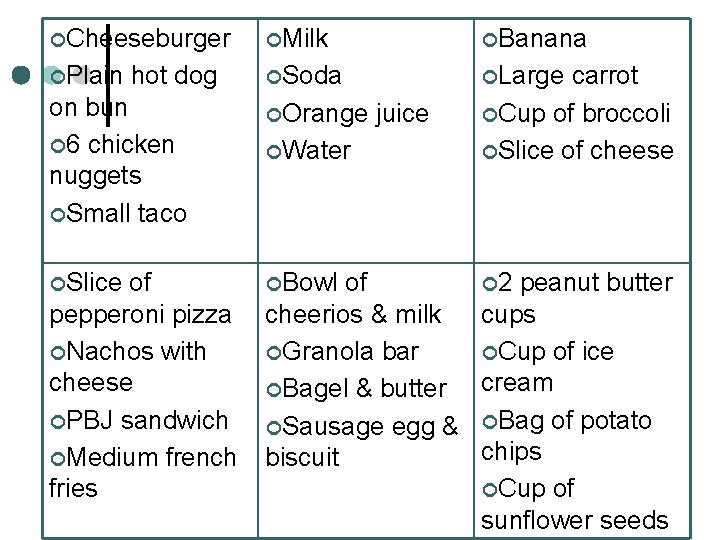
¢Cheeseburger ¢Milk ¢Banana ¢Plain ¢Soda ¢Large hot dog on bun ¢ 6 chicken nuggets ¢Small taco ¢Orange ¢Slice ¢Bowl of pepperoni pizza ¢Nachos with cheese ¢PBJ sandwich ¢Medium french fries ¢Water juice carrot ¢Cup of broccoli ¢Slice of cheese of ¢ 2 peanut butter cheerios & milk cups ¢Granola bar ¢Cup of ice cream ¢Bagel & butter ¢Sausage egg & ¢Bag of potato chips biscuit ¢Cup of sunflower seeds
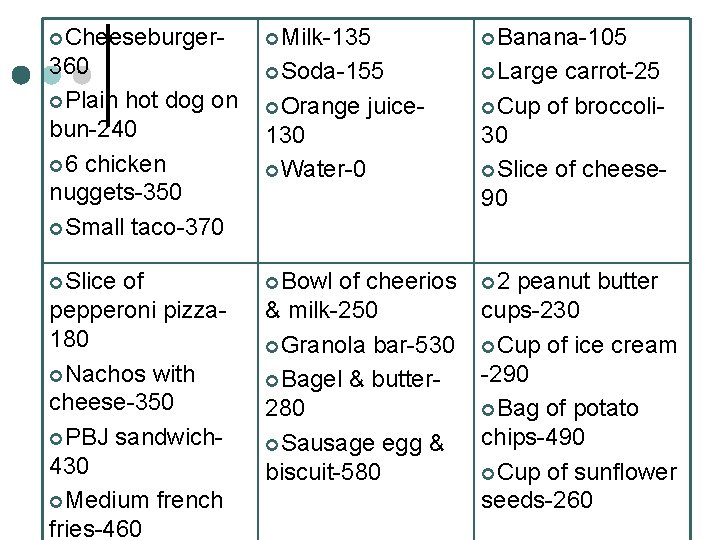
¢Cheeseburger- ¢Milk-135 ¢Banana-105 360 ¢Plain hot dog on bun-240 ¢ 6 chicken nuggets-350 ¢Small taco-370 ¢Soda-155 ¢Large ¢Slice ¢Bowl of pepperoni pizza 180 ¢Nachos with cheese-350 ¢PBJ sandwich 430 ¢Medium french fries-460 ¢Orange juice- 130 ¢Water-0 of cheerios & milk-250 ¢Granola bar-530 ¢Bagel & butter 280 ¢Sausage egg & biscuit-580 carrot-25 ¢Cup of broccoli 30 ¢Slice of cheese 90 ¢ 2 peanut butter cups-230 ¢Cup of ice cream -290 ¢Bag of potato chips-490 ¢Cup of sunflower seeds-260

Questions Into what forms of energy does you body convert food energy? ¢ What happens if your body takes in more food energy than it needs? ¢ What happens if your body does not get the food energy it needs? ¢ What other things besides energy content do you need to consider when choosing food to eat? ¢

Energy in food ¢ A 12 oz can of soda contains ~40 g of sugar. How much chemical energy in joules does this sugar contain? l How many Calories is this? l

2 nd law of thermodynamics ¢ As a result of energy transformations, disorder tends to increase l ¢ Disorder or randomness Life requires a constant input of energy to maintain order

Reactions ¢ Anabolic reactions – increase complexity (order) l Building things up • Amino acid + amino acid = dipeptide + water • Free energy + small molecules =complex molecules ¢ Catabolic reactions – break down complexity (create disorder) l Release free energy • Dipeptide energy + amino acid

Reactions ¢ Chemical reactions can run both forward and backward l Exergonic reactions – reactions that release free energy • Catabolic – breaking down a molecule • Protein to amino acids l Endergonic reactions – reactions that require or consume free energy • Anabolic – making a product out of small reactants • Amino acids to protein


Role of ATP ¢ ATP- adenosine triphosphate Energy currency l Releases large amount of energy when hydrolyzed l Phosphorylate – donate a phosphate group to many different molecules l

ATP hydrolysis releases energy Composed of adenine bonded with ribose & attached to 3 phosphate groups ¢ Hydrolysis ¢ l ATP + H 2 O ADP + Pi + free energy

Bioluminescence ¢ Endergonic reaction driven by ATP hydrolysis & interconversion of energy forms

ATP reactions ¢ Energy-coupling cycle l ADP picks up energy from exergonic reactions and becomes ATP, which donates energy to endergonic reactions
- Slides: 23