ENERGY Energy is the capacity to do work















- Slides: 30

ENERGY Energy is the capacity to do work. Energy can be broken down into kinetic and potential. Kinetic energies include the motion of particles (thermal energy), and motion of electrons through a conductor (electrical energy). Potential energies include energy stored in fuels (chemical energy), and energy between two charges (electrostatic energy). The scientific study of heat and work is called thermodynamics. This is a physics topic as well.

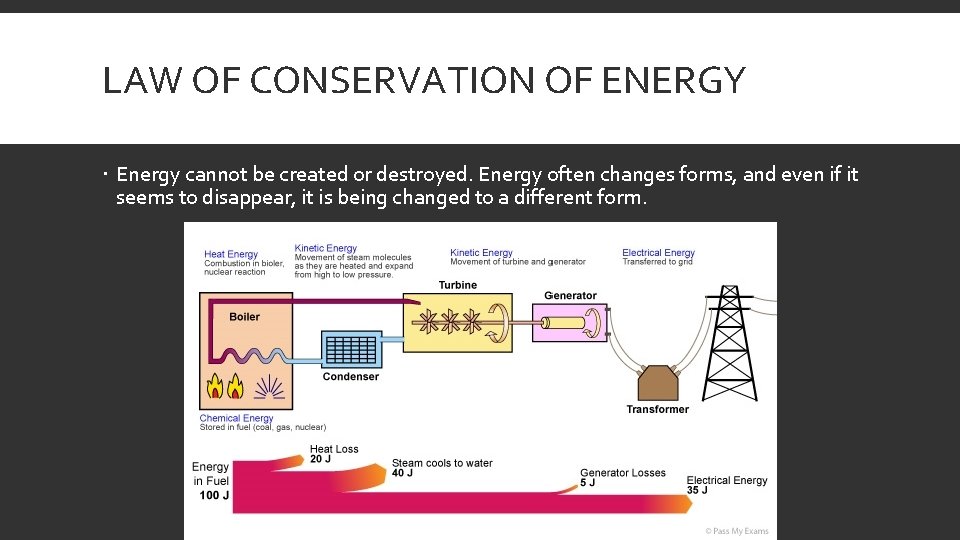
LAW OF CONSERVATION OF ENERGY Energy cannot be created or destroyed. Energy often changes forms, and even if it seems to disappear, it is being changed to a different form.

TEMPERATURE AND HEAT Temperature is a measure of the ability to transfer energy as heat. If objects are at different temperatures, thermal energy is transferred from the higher temperature to the lower temperature until they are equal temperatures. Why does a room get colder when an air conditioner blows cold air into the room? What is heat?

SYSTEM AND SURROUNDINGS In thermodynamics, we have to define what the system and surroundings are to solve some problems. The system is the object or objects that are being studied. The surroundings are everything outside the system that can exchange energy with the system. If a reaction is occurring in a beaker, what are the system and the surroundings? If we are measuring the temperature of our galaxy, what are the system and the surroundings?

THERMAL EQUILIBRIUM If two objects with different temperatures come into contact, the hotter object will cool off and the colder one will heat up until they reach the same temperature. If water at 25°C has a piece of metal dropped into it at 125°C, do you think thermal equilibrium occurs at 75°C? Why or why not? Once equilibrium is reached, transfer of energy will still occur on the molecular level, but the temperature remains constant overall.

ENERGY UNITS The SI unit of energy is the joule (J). In chemistry, we will often use kilojoules (k. J). The other common unit for energy is the calorie. It is defined as the energy required to raise the temperature of 1 gram of water one degree Celsius. Why is it just as valid to say it raises 1 gram of water one Kelvin? Calories are not the same as calories. A Calorie is actually a kilocalorie. 1 Calorie = 1000 calories 1 calorie = 4. 184 joules

PRACTICE A restaurant in Las Vegas called the Heart Attack Grill offers overly unhealthy options. They sell a burger called the Quadruple Bypass Burger that contains 9, 982 Calories. How many calories are in the burger? How many joules are in the burger? How many kilojoules are in the burger?

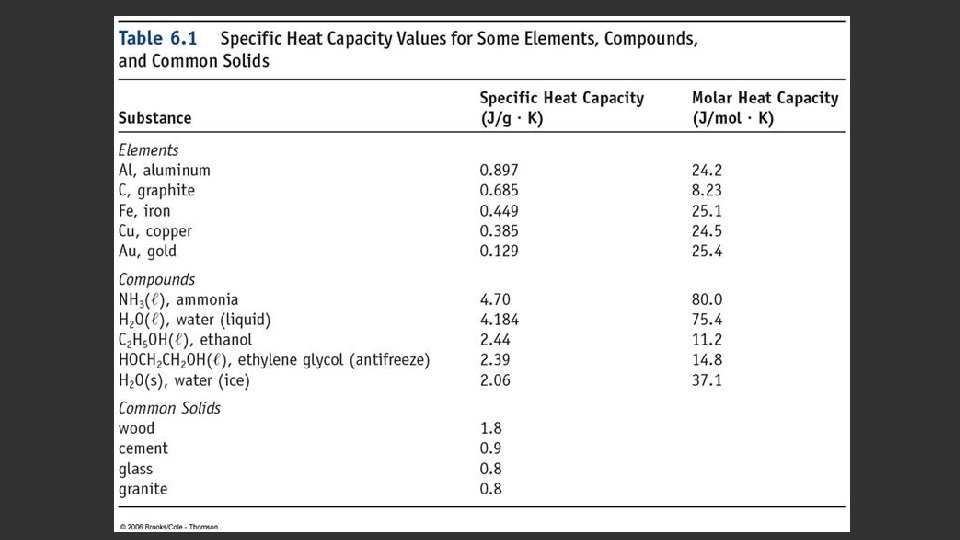
SPECIFIC HEAT CAPACITY When an object is heated or cooled, the amount of energy transferred depends on: The quantity of the material The magnitude of the temperature change The identity of the material Specific heat capacity (C) is the energy transferred as heat required to raise the temperature of 1 gram of the substance by one Kelvin. Unit: J/g·K Some specific heat capacities are listed on page 216 in the textbook. q = CmΔT heat = specific heat X mass X change in temperature


PRACTICE How much energy is transferred to raise the temperature of 250 m. L of coffee from 20. 5°C to 95. 6°C? Assume that coffee has the same density and specific heat capacity as water.

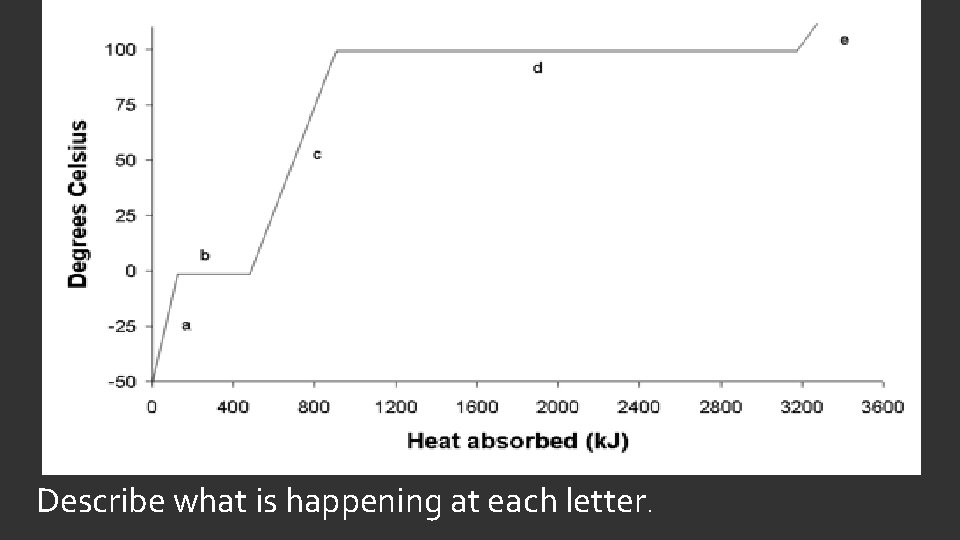
HEAT OF FUSION AND HEAT OF VAPORIZATION When a substance is changing its state, it is absorbing heat even though the temperature is constant. The amount of energy required to change from solid to liquid is the heat of fusion. The amount of energy required to change from liquid to gas is the heat of vaporization. Values for both are found in Appendix D (Table 12). Waters heat of fusion is 333 J/g and its heat of vaporization is 2256 J/g. To find the amount of energy, simply multiply by the mass of the sample.

Describe what is happening at each letter.

EXAMPLE Calculate the energy required to convert 500. g of ice at -50. 0°C to steam at 200. °C.

ANOTHER EXAMPLE What is the minimum amount of ice at 0°C that must be added to a can of diet cola (340. m. L) to cool the cola from 20. 5°C to 0. 0°C? Assume water has the same specific heat and density as the diet cola.

FIRST LAW OF THERMODYNAMICS The change in energy for a system is equal to the energy transferred as heat plus the work done. ΔU = q + w Heat and work could both be added or removed from the system. When added, the energy increases and when it is removed the energy decreases. If dry ice (solid CO 2) is placed in a sealed bag, as sublimation occurs, the solid particles absorb energy to become gas and the system does work by expanding the bag. U is the symbol for internal energy, the sum of potential and kinetic energies of the atoms, molecules, or ions in the system.

ENTHALPY Enthalpy is the energy measured at a constant pressure. Most experiments occur at constant pressure. The symbol used for enthalpy is H. ΔH = ΔU + PΔV Enthalpy is equal to the internal energy plus the work. When enthalpy is negative, the system transfers heat to the surroundings. When enthalpy is positive, the surroundings transfer heat to the system.

STATE FUNCTIONS Internal energy and enthalpy are both state functions. That is their values only depend on their initial and final states. ΔH is always the same for a given set of reactants and products. You can use different reactions to obtain the products, but the ΔH will be the same regardless. There are many ways to travel from Orlando to Tampa, but no matter which route you take, you are still 77 miles from where you began.

ENTHALPY CHANGES FOR REACTIONS Values for standard reaction enthalpy can be looked up, but in this book they are given in the problem. They are standard, which means they are all measured at a pressure of 1 bar and 25°C. H 2 O(g) → H 2(g) + ½ O 2(g) Δr. H° = +241. 8 k. J/mol-rxn Moles of reaction is the denominator in the unit. A mole of reaction has occurred when 1 mole of water makes 1 mole of hydrogen and one-half of a mole of oxygen. Predict the Δr. H° for each of the following reactions: H 2(g) + ½ O 2(g) → H 2 O(g) 2 H 2(g) + O 2(g) → 2 H 2 O(g)

CONTINUED Make sure you use the correct states of matter. H 2(g) + ½ O 2(g) → H 2 O(l) Δr. H° = -285. 8 k. J/mol-rxn What would be the sign of enthalpy for an exothermic reaction? What would be the sign of enthalpy for an endothermic reaction?

CALCULATING ENTHALPY Calculate the energy transferred as heat when 15. 0 g of C 2 H 6 are burned. 2 C 2 H 6(g) + 7 O 2(g) → 4 CO 2(g) + 6 H 2 O(g) Δr. H° = -2857. 3 k. J/mol-rxn Calculate the energy transferred as heat when 15. 0 g of O 2 are burned.

COFFEE-CUP CALORIMETRY qr + qsolution = 0 If you place 0. 05 oo g magnesium in a calorimeter and add 100 m. L of 1. 00 M HCl. Mg(s) + 2 HCl(aq) → H 2(g) + Mg. Cl 2(aq) The temperature increases from 22. 21°C to 24. 46°C. What is the enthalpy change per mol of Mg? The specific heat is 4. 20 J/g·K and the density of HCl is 1. 00 g/m. L.

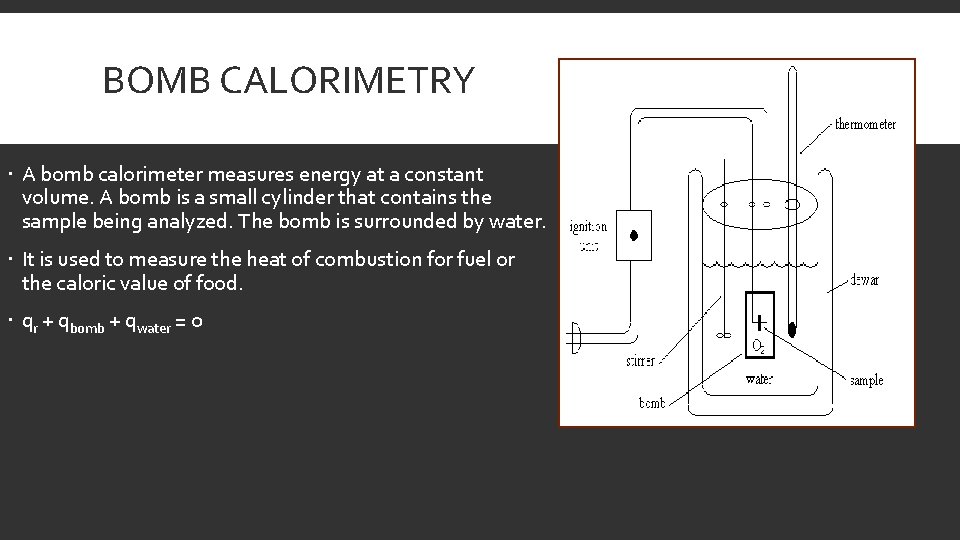
BOMB CALORIMETRY A bomb calorimeter measures energy at a constant volume. A bomb is a small cylinder that contains the sample being analyzed. The bomb is surrounded by water. It is used to measure the heat of combustion for fuel or the caloric value of food. qr + qbomb + qwater = 0

EXAMPLE C 8 H 18(l) + 25/2 o 2(g) → 8 CO 2(g) + 9 H 2 O(l) 1. 00 g of octane are burned in a bomb calorimeter with 1. 20 kg of water surrounding it. The temperature of the bomb and water rise from 25. 00 °C to 33. 20 °C. The heat capacity of the bomb is 837 J/K. What is the heat of combustion per gram of octane? What is the heat of combustion per mole?

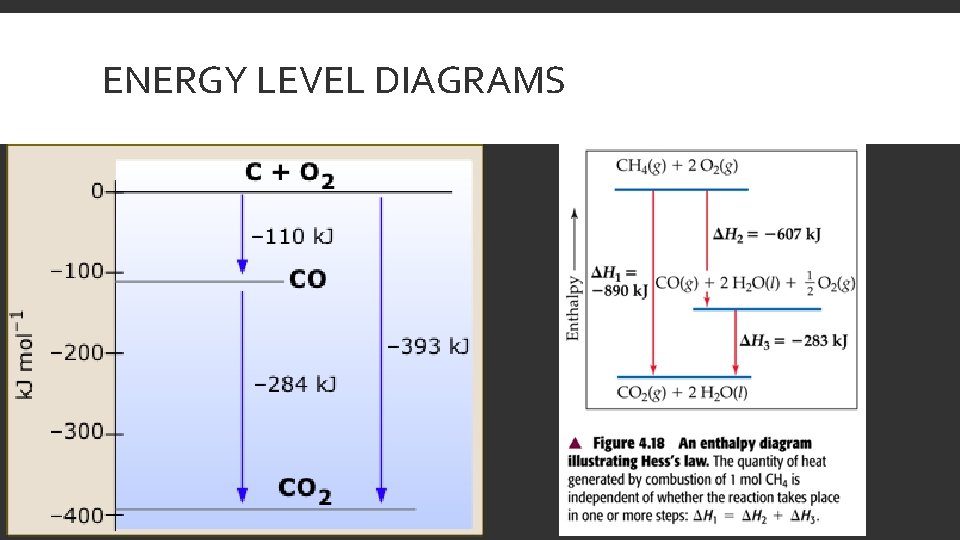
HESS’S LAW A reaction that is a combination of two reactions has a Δr. H° equal to the sum of the Δr. H° for each reaction The oxidation of graphite to make CO 2 is the combination of two reactions. C(graphite) + ½ O 2(g) → CO(g) Δr. H° = ? CO(g) + ½ O 2(g) → CO 2(g) Δr. H° = -283. 0 k. J/mol·rxn Δr. H° = - 393. 5 k. J/mol·rxn

ENERGY LEVEL DIAGRAMS

EXAMPLE Find Δr. H° for C(s) + 2 H 2(g) → CH 4(g) C(s) + O 2(g) → CO 2(g) H 2(g) + ½ O 2 → H 2 O(l) Δr. H° = -393. 5 k. J/mol·rxn Δr. H° = -285. 8 k. J/mol·rxn CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(l) Δr. H° = -890. 3 k. J/mol·rxn

PRACTICE Calculate Δr. H° for C(s) + 2 S(s) → CS 2(l) C(s) + O 2(g) → CO 2(g) Δr. H° = -393. 5 k. J/mol·rxn S(s) + O 2(g) → SO 2(g) Δr. H° = -295. 8 k. J/mol·rxn CS 2(l) + 3 O 2(g) → CO 2(g) + 2 SO 2(g) Δr. H° = -1103. 9 k. J/mol·rxn

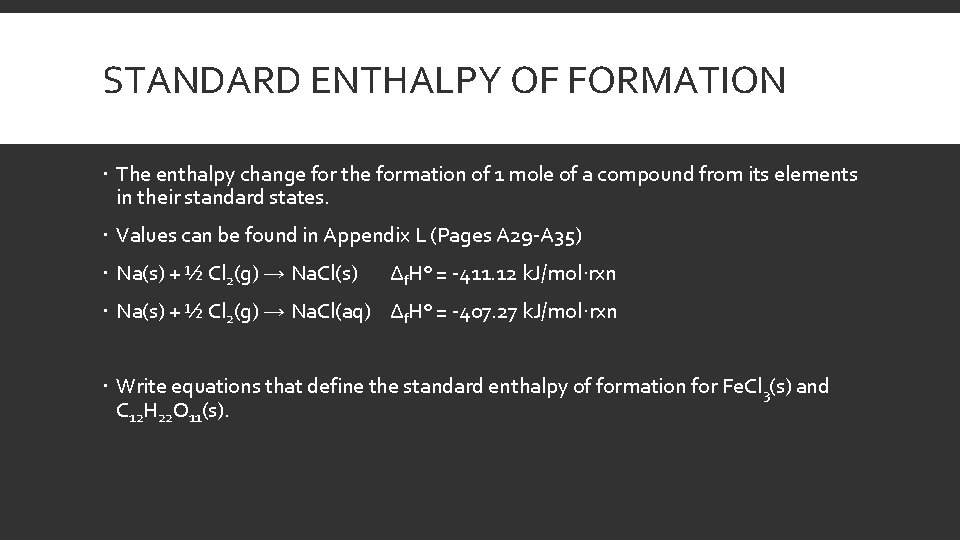
STANDARD ENTHALPY OF FORMATION The enthalpy change for the formation of 1 mole of a compound from its elements in their standard states. Values can be found in Appendix L (Pages A 29 -A 35) Na(s) + ½ Cl 2(g) → Na. Cl(s) Δf. H° = -411. 12 k. J/mol·rxn Na(s) + ½ Cl 2(g) → Na. Cl(aq) Δf. H° = -407. 27 k. J/mol·rxn Write equations that define the standard enthalpy of formation for Fe. Cl 3(s) and C 12 H 22 O 11(s).

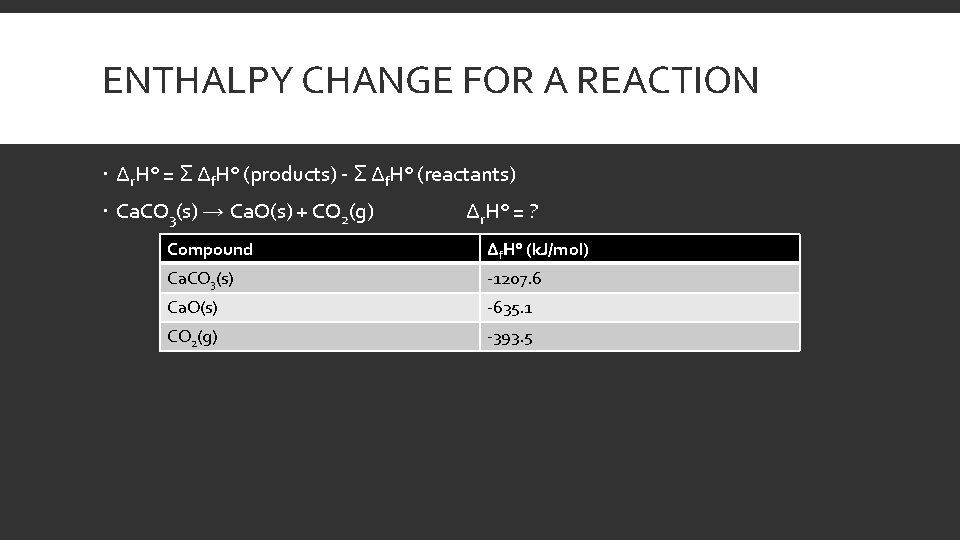
ENTHALPY CHANGE FOR A REACTION Δr. H° = Σ Δf. H° (products) - Σ Δf. H° (reactants) Ca. CO 3(s) → Ca. O(s) + CO 2(g) Δr. H° = ? Compound Δf. H° (k. J/mol) Ca. CO 3(s) -1207. 6 Ca. O(s) -635. 1 CO 2(g) -393. 5

PRACTICE Calculate the standard enthalpy of combustion for benzene, C 6 H 6. Δf. H°[C 6 H 6] = + 49. 0 k. J/mol