Energy ENERGY Energy the ability to do work
Energy

ENERGY • Energy- the ability to do work – SI unit for energy is Joules (J) – 2 basic forms • Potential energy- energy of position • Kinetic energy- energy of motion • Energy is classified by its source – Mechanical potential energy • Common example is gravitational potential energy

Energy • Gravitational potential energy – GPE= mgh – EX. How high has a 7. 26 kg shot put been thrown if it has a potential energy of 240. 2 J? – h= GPE/(mg) • • GPE=240. 2 J m= 7. 26 kg g= 9. 81 m/s 2 h=? – h= 240. 2 J/ [(7. 26 kg)(9. 81 m/s 2)] – h= 3. 37 m
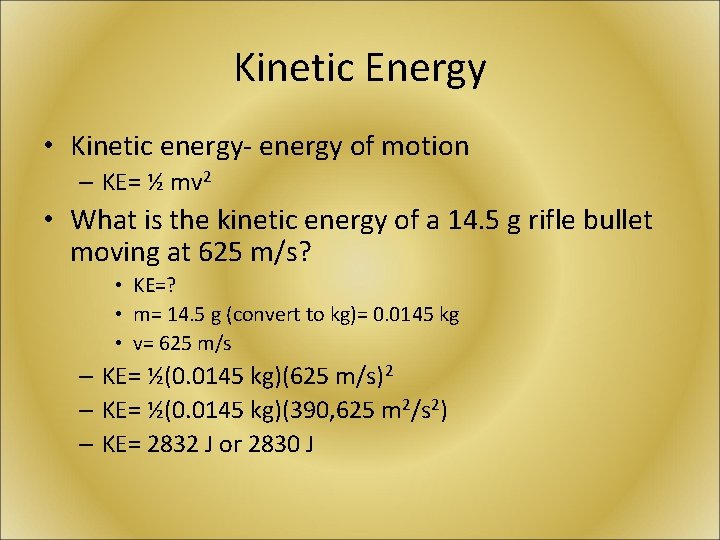
Kinetic Energy • Kinetic energy- energy of motion – KE= ½ mv 2 • What is the kinetic energy of a 14. 5 g rifle bullet moving at 625 m/s? • KE=? • m= 14. 5 g (convert to kg)= 0. 0145 kg • v= 625 m/s – KE= ½(0. 0145 kg)(625 m/s)2 – KE= ½(0. 0145 kg)(390, 625 m 2/s 2) – KE= 2832 J or 2830 J

Chapter 6 Energy • Thermal Energy- the sum of all of the kinetic energies of all the particles in an object • Theoretically, At 0 Kelvin all particle movement stops, but this is not achievable on earth • Acoustic Energy- involves random vibrations and motion of particles • Produces sound • must travel through matter-cannot exist in a vacuum • Moves in periodic motion- back and forth vibrations

Energy • Electrical Energy- forces acting on other electrical charges • Opposite attract, like repel • Natural sources- lightning, electric rays and eels • Man made sources- batteries, generators, etc • Magnetic Energy-ability to do work through the influence of a magnetic field – Opposite attract, like repel • Radiant Energy- also called electromagnetic energy- does not require matter through which to travel, can travel in a vacuum – Most common form is visible light

Energy • Chemical Energy- potential energy stored in chemical bonds – During chemical reactions atoms rearrange their bonds and energy is released or absorbed – Energy can be released as thermal, radiant, or acoustic energy – Note: we will go over this in more detail in Chapters 18 and 19

Energy • Nuclear Energy- this is the energy associated with the nucleus of an atom – Can be released in 2 ways • Fusion- is the combining of 2 or more nuclei to form a larger nuclei – stars 2 H atoms combine to form He • Fission- is the splitting apart of a nucleus into 2 more smaller nuclei – Man made nuclear power plants to generate power Nuclear energy always releases radiation-DANGER REMEMBER- energy cannot be created nor destroyed only transferred- all energies in the end must equal that with which you started!!

Energy • Mass Energy- this is the largest source of potential energy in the universe – It is the energy equivalent to all matter itself – This is where Einstein’s theory is applied • E= mc 2 • Energy is equal to the mass times the speed of light squared
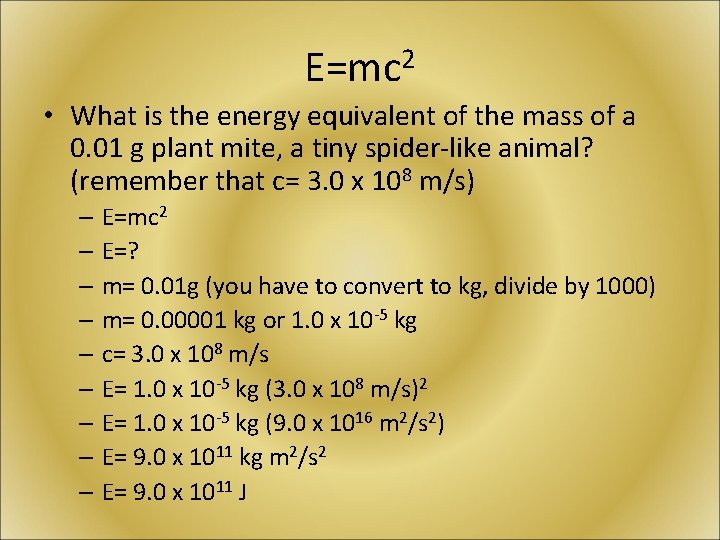
E=mc 2 • What is the energy equivalent of the mass of a 0. 01 g plant mite, a tiny spider-like animal? (remember that c= 3. 0 x 108 m/s) – E=mc 2 – E=? – m= 0. 01 g (you have to convert to kg, divide by 1000) – m= 0. 00001 kg or 1. 0 x 10 -5 kg – c= 3. 0 x 108 m/s – E= 1. 0 x 10 -5 kg (3. 0 x 108 m/s)2 – E= 1. 0 x 10 -5 kg (9. 0 x 1016 m 2/s 2) – E= 9. 0 x 1011 kg m 2/s 2 – E= 9. 0 x 1011 J

Example 6 -3 • How much mass energy, in joules, could be obtained from the complete conversion of a compact 138 g media player? – m= 138 g = 0. 138 kg – c= 3. 00 x 108 m/s 2 – E= ? – E= mc 2 E= 0. 138 kg (3. 00 x 108 m/s)2 E= 1. 242 x 1016 kg* m 2/s 2 E= 1. 24 x 1016 J
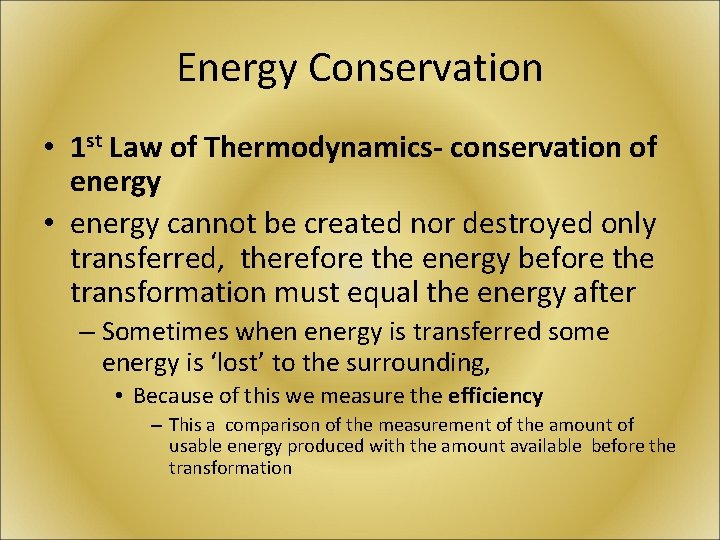
Energy Conservation • 1 st Law of Thermodynamics- conservation of energy • energy cannot be created nor destroyed only transferred, therefore the energy before the transformation must equal the energy after – Sometimes when energy is transferred some energy is ‘lost’ to the surrounding, • Because of this we measure the efficiency – This a comparison of the measurement of the amount of usable energy produced with the amount available before the transformation
Energy Conservation • Energy can be transferred to one or more forms of energy – Chemical energy from food becomes thermal energy in our cells and mechanical energy in our muscles • The 1 st Law of thermodynamics is easily demonstrated by a pendulum – Consists of a heavy weight at the end of an arm that swings back and forth on a pivot point at its upper end

Collisions and Energy • As we have discussed before momentum and kinetic energy are properties of motion • Momentum is a vector defining a system’s quantity of motion • Kinetic energy is a scalar quantity describing the mechanical energy of a moving system • Collisions…………….

Collisons • There are 3 types of collisions – 1. Elastic collisions- occurs when 2 objects collide and bounce (rebound) off from one another • The sum of their momentums and the sum of their kinetic energies are the same before and after the collision – 2. Partially elastic collisions – occurs when one or both objects in a collision are deformed before rebounding – 3. Inelastic collision- occurs when two objects collide and stick together REMEMBER ENERGY IS NOT LOST BUT TRANSFERRED
- Slides: 15