Energy changes in reactions Exothermic and Endothermic what
- Slides: 19

Energy changes in reactions Exothermic and Endothermic – what do they mean? * ** *** To sort a reaction into endothermic or exothermic based on own results To describe the properties of an endothermic and exothermic reaction To explain what is happening in an endothermic and exothermic reaction

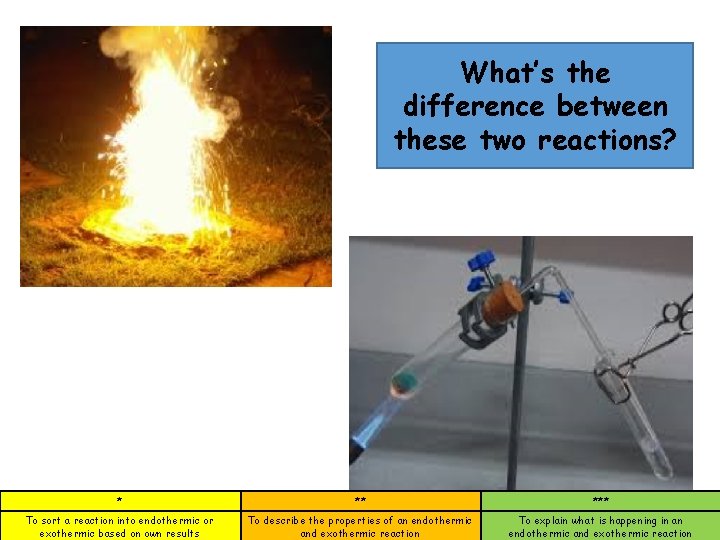
What’s the difference between these two reactions? * ** *** To sort a reaction into endothermic or exothermic based on own results To describe the properties of an endothermic and exothermic reaction To explain what is happening in an endothermic and exothermic reaction

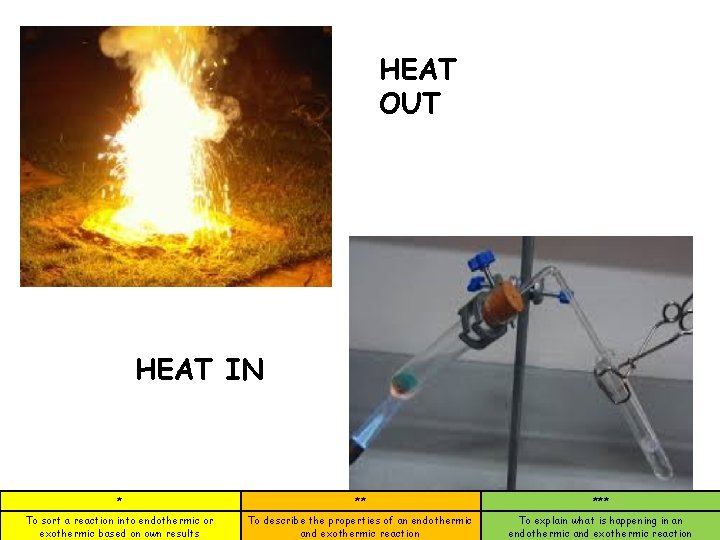
HEAT OUT HEAT IN * ** *** To sort a reaction into endothermic or exothermic based on own results To describe the properties of an endothermic and exothermic reaction To explain what is happening in an endothermic and exothermic reaction

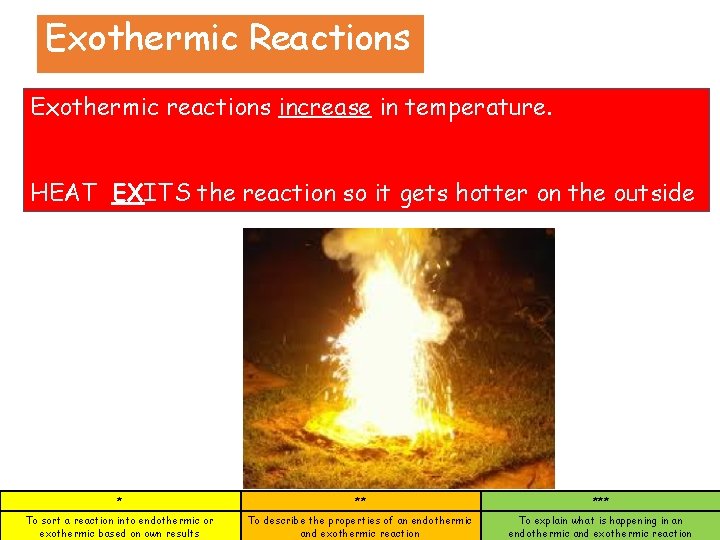
Exothermic Reactions Exothermic reactions increase in temperature. HEAT EXITS the reaction so it gets hotter on the outside * ** *** To sort a reaction into endothermic or exothermic based on own results To describe the properties of an endothermic and exothermic reaction To explain what is happening in an endothermic and exothermic reaction

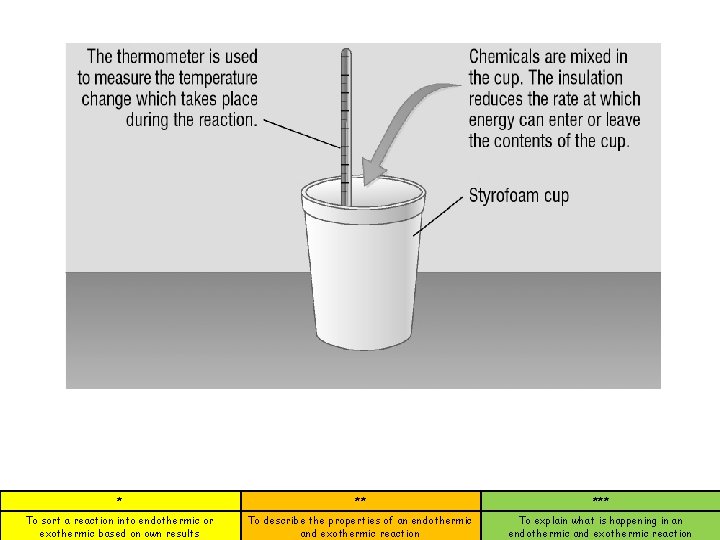
Endothermic Reactions Endothermic reactions decrease in temperature. HEAT Enters the reaction so it gets colder on the outside. * ** *** To sort a reaction into endothermic or exothermic based on own results To describe the properties of an endothermic and exothermic reaction To explain what is happening in an endothermic and exothermic reaction

* ** *** To sort a reaction into endothermic or exothermic based on own results To describe the properties of an endothermic and exothermic reaction To explain what is happening in an endothermic and exothermic reaction

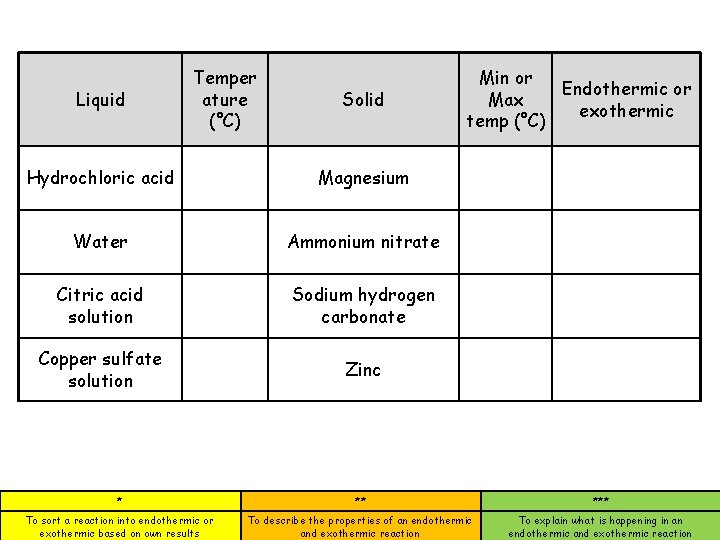
Liquid Temper ature (°C) Solid Hydrochloric acid Magnesium Water Ammonium nitrate Citric acid solution Sodium hydrogen carbonate Copper sulfate solution Zinc Min or Endothermic or Max exothermic temp (°C) * ** *** To sort a reaction into endothermic or exothermic based on own results To describe the properties of an endothermic and exothermic reaction To explain what is happening in an endothermic and exothermic reaction

Summary Q’s • Name an example of an exothermic reaction • Name an example of an endothermic reaction • What happens to the energy in an endothermic reaction? How do you know? • What happens to the energy in an exothermic reaction? How do you know? * ** *** To sort a reaction into endothermic or exothermic based on own results To describe the properties of an endothermic and exothermic reaction To explain what is happening in an endothermic and exothermic reaction

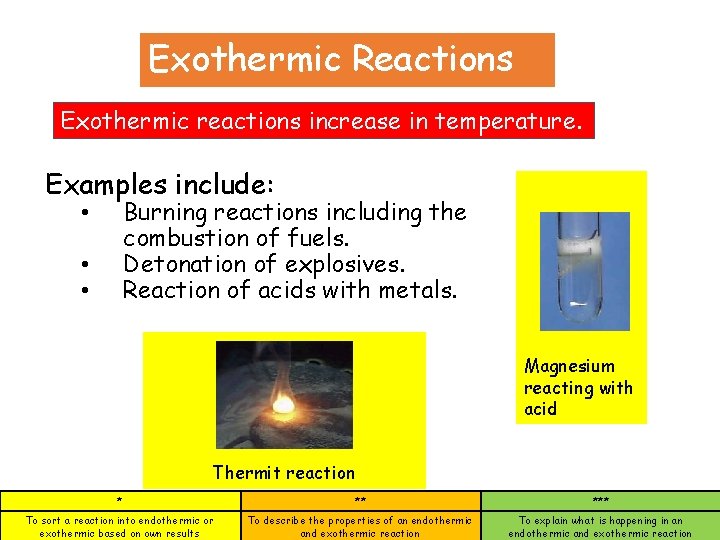
Exothermic Reactions Exothermic reactions increase in temperature. Examples include: Burning reactions including the combustion of fuels. Detonation of explosives. Reaction of acids with metals. • • • Magnesium reacting with acid Thermit reaction * ** *** To sort a reaction into endothermic or exothermic based on own results To describe the properties of an endothermic and exothermic reaction To explain what is happening in an endothermic and exothermic reaction

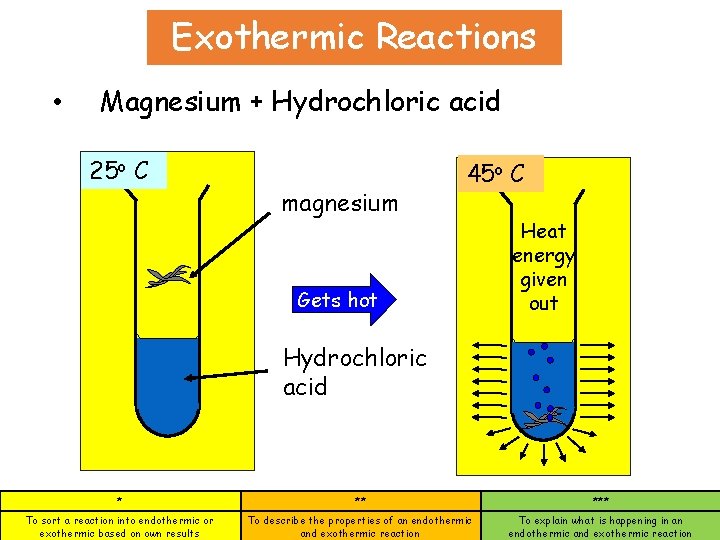
Exothermic Reactions • Magnesium + Hydrochloric acid 25 o C magnesium 45 o C Gets hot Heat energy given out Hydrochloric acid * ** *** To sort a reaction into endothermic or exothermic based on own results To describe the properties of an endothermic and exothermic reaction To explain what is happening in an endothermic and exothermic reaction

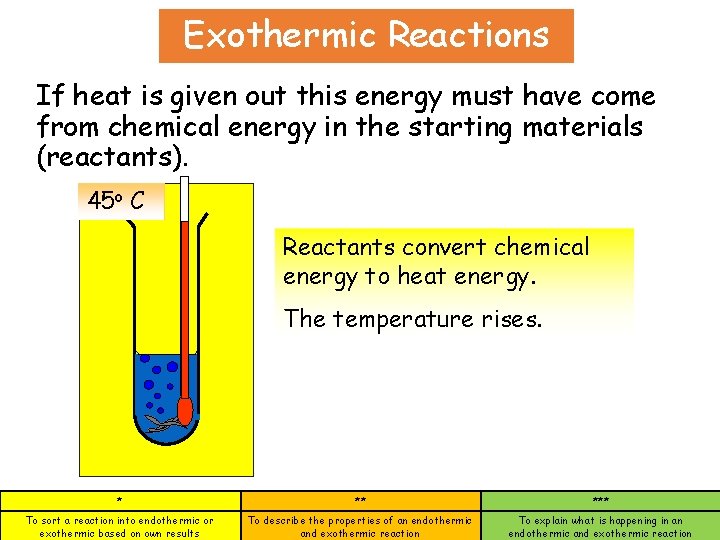
Exothermic Reactions If heat is given out this energy must have come from chemical energy in the starting materials (reactants). 45 25 o C Reactants convert chemical energy to heat energy. The temperature rises. * ** *** To sort a reaction into endothermic or exothermic based on own results To describe the properties of an endothermic and exothermic reaction To explain what is happening in an endothermic and exothermic reaction

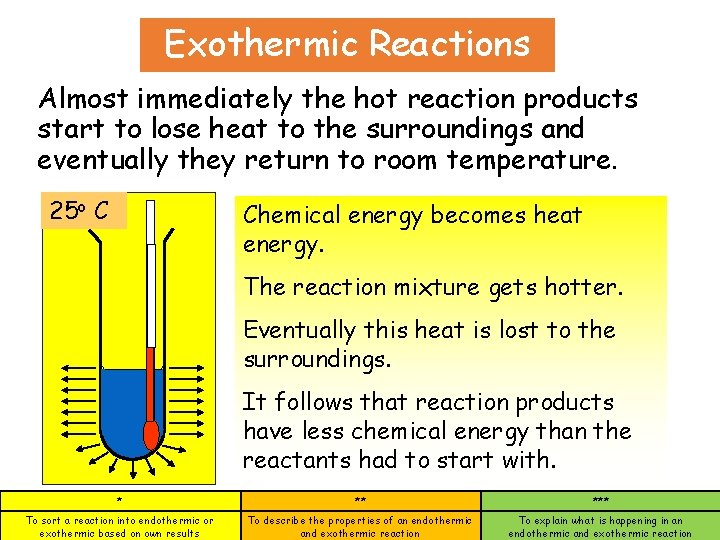
Exothermic Reactions Almost immediately the hot reaction products start to lose heat to the surroundings and eventually they return to room temperature. 25 o C 45 Chemical energy becomes heat energy. The reaction mixture gets hotter. Eventually this heat is lost to the surroundings. It follows that reaction products have less chemical energy than the reactants had to start with. * ** *** To sort a reaction into endothermic or exothermic based on own results To describe the properties of an endothermic and exothermic reaction To explain what is happening in an endothermic and exothermic reaction

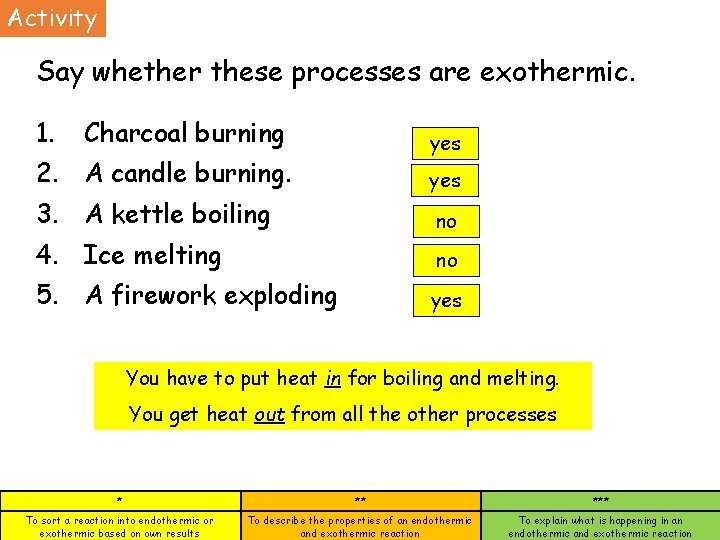
Activity Say whether these processes are exothermic. 1. Charcoal burning yes 2. A candle burning. yes 3. A kettle boiling no 4. Ice melting no 5. A firework exploding yes You have to put heat in for boiling and melting. You get heat out from all the other processes * ** *** To sort a reaction into endothermic or exothermic based on own results To describe the properties of an endothermic and exothermic reaction To explain what is happening in an endothermic and exothermic reaction

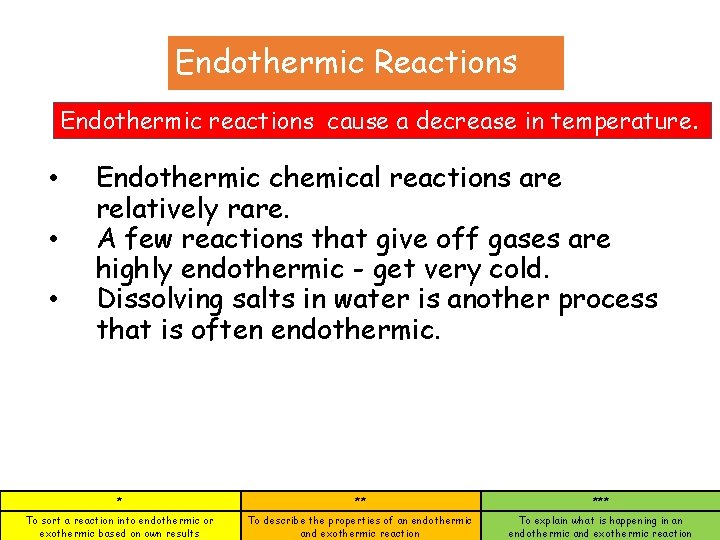
Endothermic Reactions Endothermic reactions cause a decrease in temperature. • • • Endothermic chemical reactions are relatively rare. A few reactions that give off gases are highly endothermic - get very cold. Dissolving salts in water is another process that is often endothermic. * ** *** To sort a reaction into endothermic or exothermic based on own results To describe the properties of an endothermic and exothermic reaction To explain what is happening in an endothermic and exothermic reaction

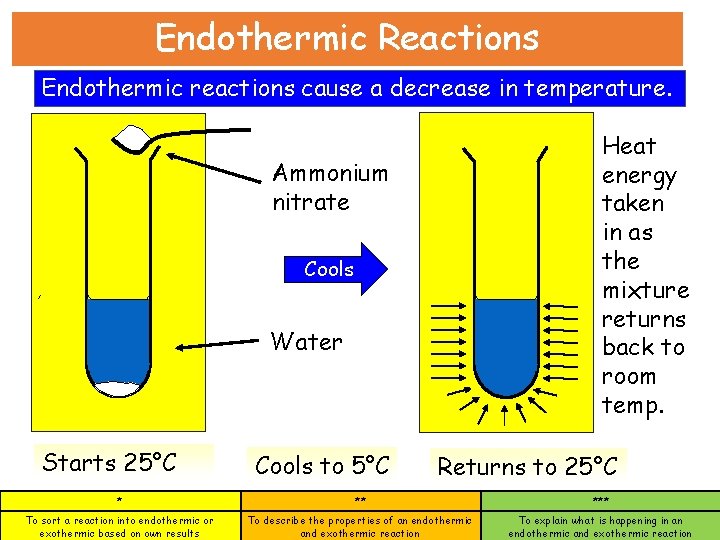
Endothermic Reactions Endothermic reactions cause a decrease in temperature. Heat energy taken in as the mixture returns back to room temp. Ammonium nitrate Cools Water Starts 25°C Cools to 5°C Returns to 25°C * ** *** To sort a reaction into endothermic or exothermic based on own results To describe the properties of an endothermic and exothermic reaction To explain what is happening in an endothermic and exothermic reaction

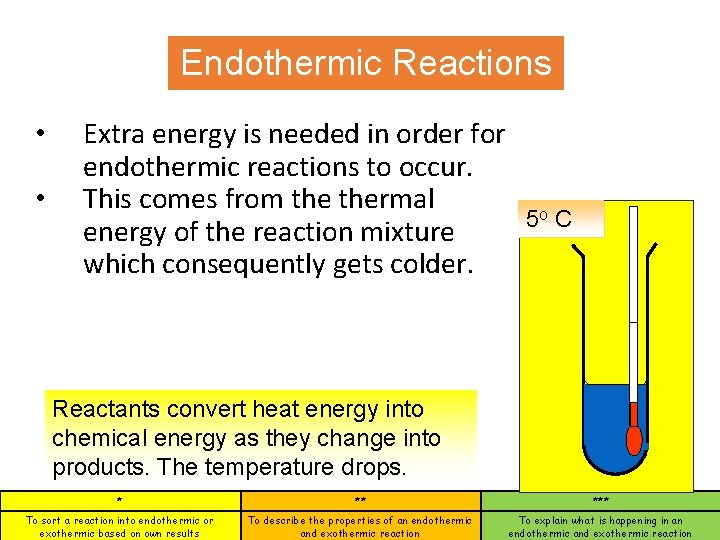
Endothermic Reactions • • Extra energy is needed in order for endothermic reactions to occur. This comes from thermal energy of the reaction mixture which consequently gets colder. oo 5 CC 25 Reactants convert heat energy into chemical energy as they change into products. The temperature drops. * ** *** To sort a reaction into endothermic or exothermic based on own results To describe the properties of an endothermic and exothermic reaction To explain what is happening in an endothermic and exothermic reaction

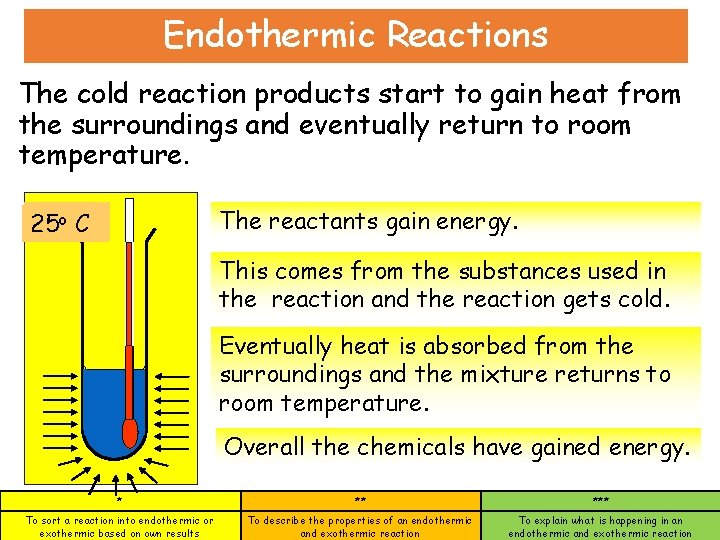
Endothermic Reactions The cold reaction products start to gain heat from the surroundings and eventually return to room temperature. The reactants gain energy. o o. C C 25 5 This comes from the substances used in the reaction and the reaction gets cold. Eventually heat is absorbed from the surroundings and the mixture returns to room temperature. Overall the chemicals have gained energy. * ** *** To sort a reaction into endothermic or exothermic based on own results To describe the properties of an endothermic and exothermic reaction To explain what is happening in an endothermic and exothermic reaction

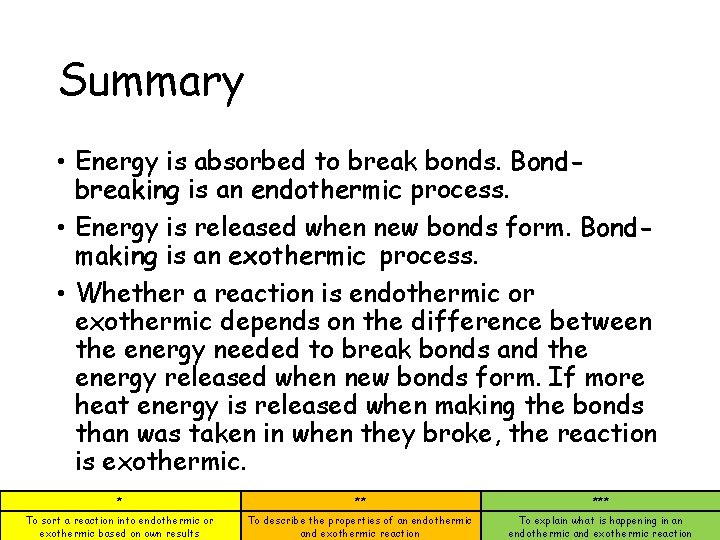
Summary • Energy is absorbed to break bonds. Bondbreaking is an endothermic process. • Energy is released when new bonds form. Bondmaking is an exothermic process. • Whether a reaction is endothermic or exothermic depends on the difference between the energy needed to break bonds and the energy released when new bonds form. If more heat energy is released when making the bonds than was taken in when they broke, the reaction is exothermic. * ** *** To sort a reaction into endothermic or exothermic based on own results To describe the properties of an endothermic and exothermic reaction To explain what is happening in an endothermic and exothermic reaction

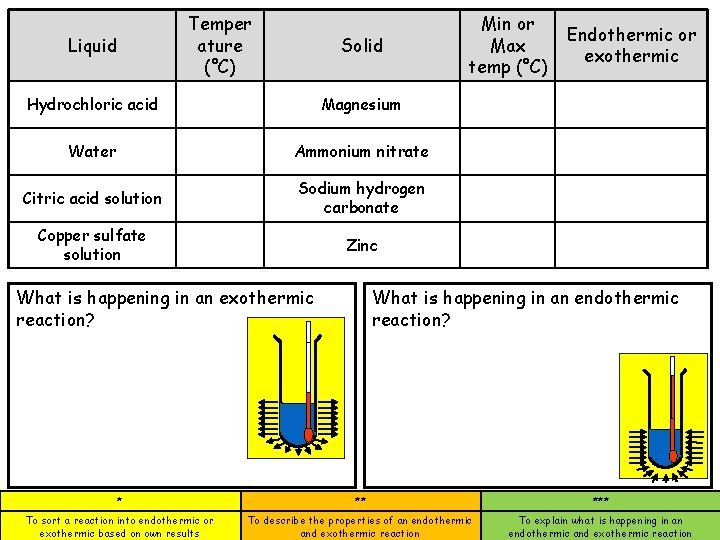
Temper ature (°C) Liquid Solid Hydrochloric acid Magnesium Water Ammonium nitrate Citric acid solution Sodium hydrogen carbonate Copper sulfate solution Zinc Min or Max temp (°C) Endothermic or exothermic What is happening in an endothermic reaction? What is happening in an exothermic reaction? * ** *** To sort a reaction into endothermic or exothermic based on own results To describe the properties of an endothermic and exothermic reaction To explain what is happening in an endothermic and exothermic reaction