Energy Balance of Reactive Systems Chapter 9 Types

Energy Balance of Reactive Systems Chapter 9
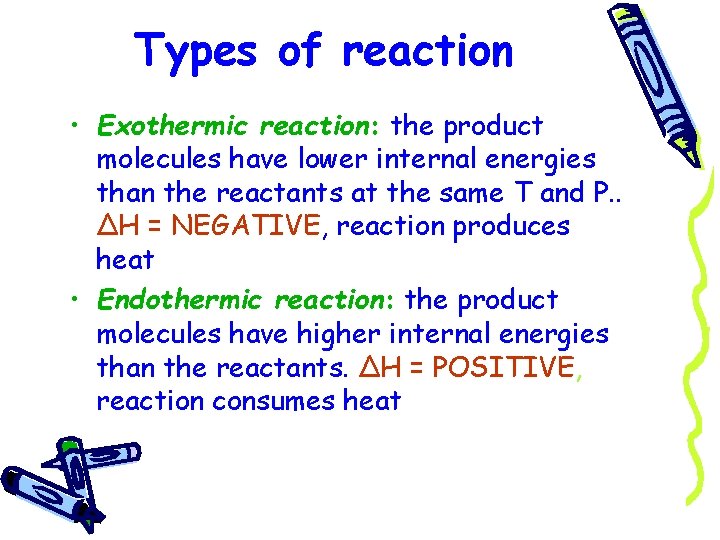
Types of reaction • Exothermic reaction: the product molecules have lower internal energies than the reactants at the same T and P. . ΔH = NEGATIVE, reaction produces heat • Endothermic reaction: the product molecules have higher internal energies than the reactants. ΔH = POSITIVE, reaction consumes heat
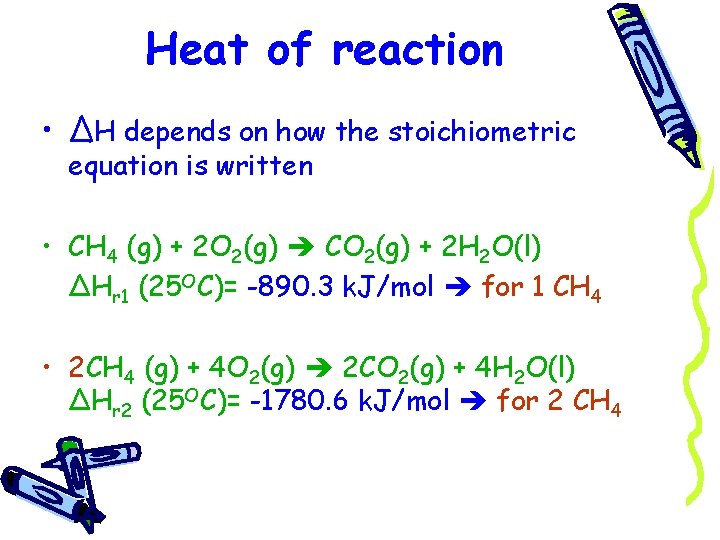
Heat of reaction • ΔH depends on how the stoichiometric equation is written • CH 4 (g) + 2 O 2(g) CO 2(g) + 2 H 2 O(l) ΔHr 1 (25 OC)= -890. 3 k. J/mol for 1 CH 4 • 2 CH 4 (g) + 4 O 2(g) 2 CO 2(g) + 4 H 2 O(l) ΔHr 2 (25 OC)= -1780. 6 k. J/mol for 2 CH 4
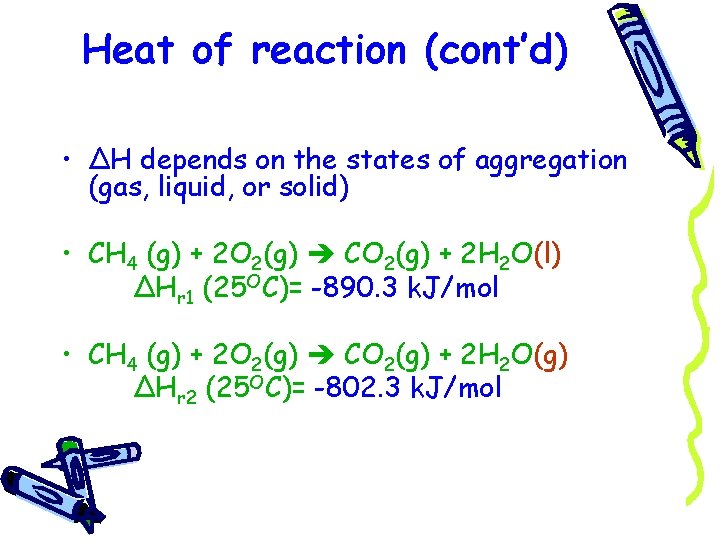
Heat of reaction (cont’d) • ΔH depends on the states of aggregation (gas, liquid, or solid) • CH 4 (g) + 2 O 2(g) CO 2(g) + 2 H 2 O(l) ΔHr 1 (25 OC)= -890. 3 k. J/mol • CH 4 (g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) ΔHr 2 (25 OC)= -802. 3 k. J/mol
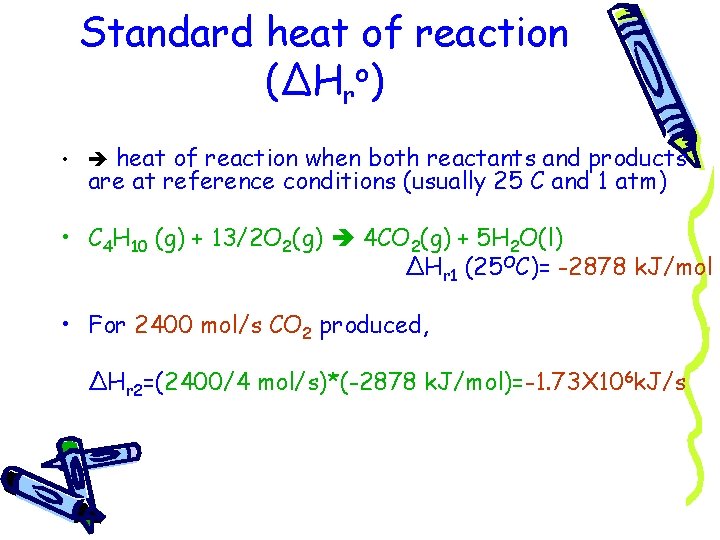
Standard heat of reaction (ΔHro) • heat of reaction when both reactants and products are at reference conditions (usually 25 C and 1 atm) • C 4 H 10 (g) + 13/2 O 2(g) 4 CO 2(g) + 5 H 2 O(l) ΔHr 1 (25 OC)= -2878 k. J/mol • For 2400 mol/s CO 2 produced, ΔHr 2=(2400/4 mol/s)*(-2878 k. J/mol)=-1. 73 X 106 k. J/s
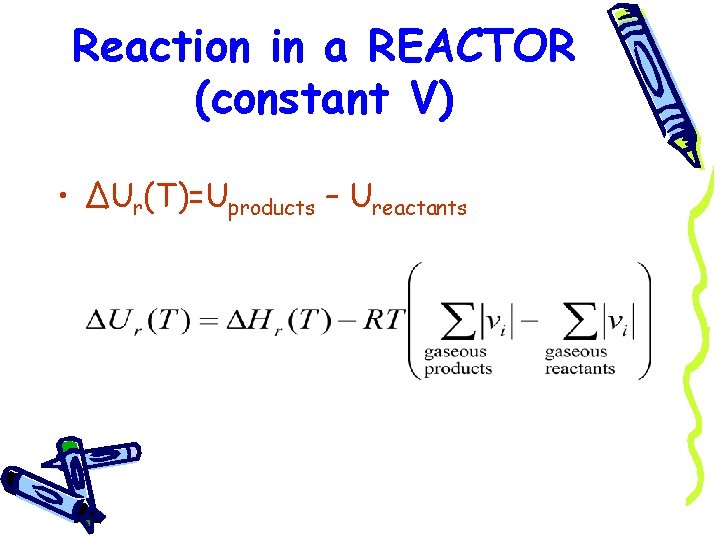
Reaction in a REACTOR (constant V) • ΔUr(T)=Uproducts – Ureactants
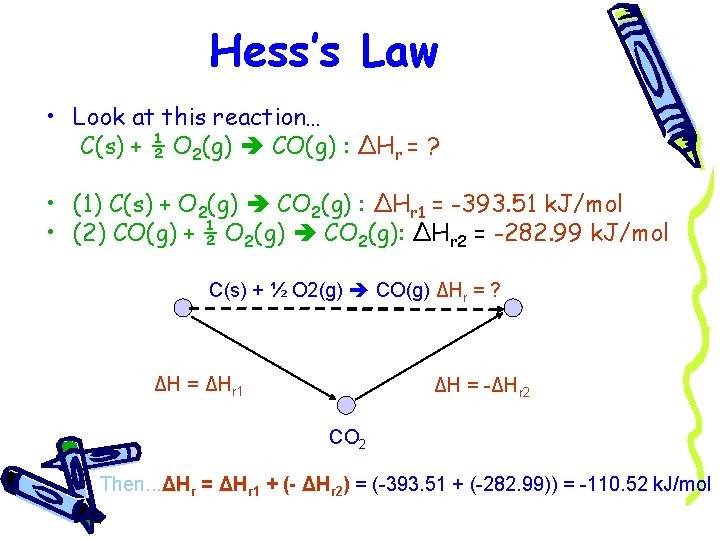
Hess’s Law • Look at this reaction… C(s) + ½ O 2(g) CO(g) : ΔHr = ? • (1) C(s) + O 2(g) CO 2(g) : ΔHr 1 = -393. 51 k. J/mol • (2) CO(g) + ½ O 2(g) CO 2(g): ΔHr 2 = -282. 99 k. J/mol C(s) + ½ O 2(g) CO(g) ΔHr = ? ΔH = ΔHr 1 ΔH = -ΔHr 2 CO 2 Then. . . ΔHr = ΔHr 1 + (- ΔHr 2) = (-393. 51 + (-282. 99)) = -110. 52 k. J/mol
Working Session Example 9. 2 -1
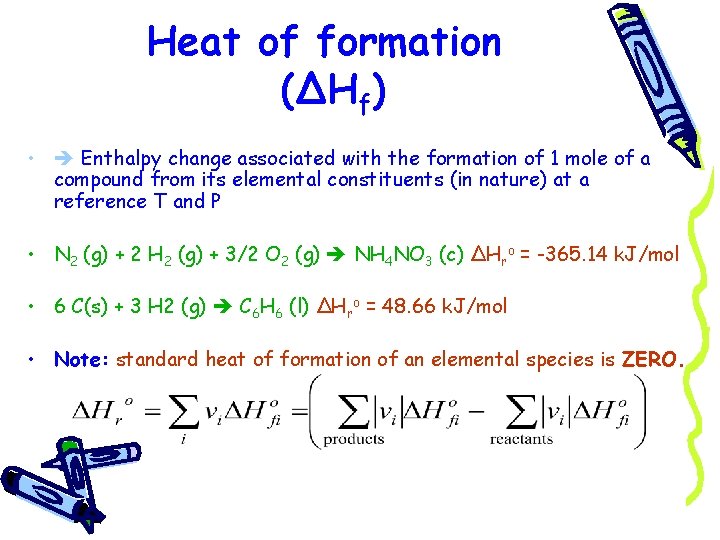
Heat of formation (ΔHf) • Enthalpy change associated with the formation of 1 mole of a compound from its elemental constituents (in nature) at a reference T and P • N 2 (g) + 2 H 2 (g) + 3/2 O 2 (g) NH 4 NO 3 (c) ΔHro = -365. 14 k. J/mol • 6 C(s) + 3 H 2 (g) C 6 H 6 (l) ΔHro = 48. 66 k. J/mol • Note: standard heat of formation of an elemental species is ZERO.
Working Session Example 9. 3 -1
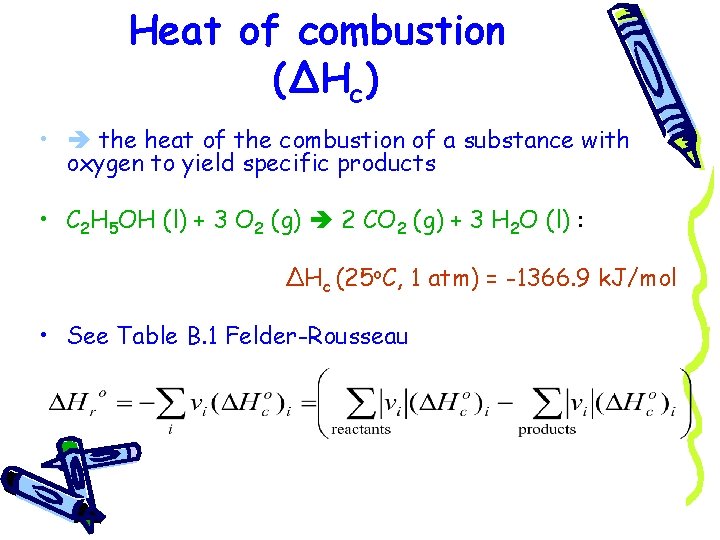
Heat of combustion (ΔHc) • the heat of the combustion of a substance with oxygen to yield specific products • C 2 H 5 OH (l) + 3 O 2 (g) 2 CO 2 (g) + 3 H 2 O (l) : ΔHc (25 o. C, 1 atm) = -1366. 9 k. J/mol • See Table B. 1 Felder-Rousseau
Working Session Example 9. 4 -1
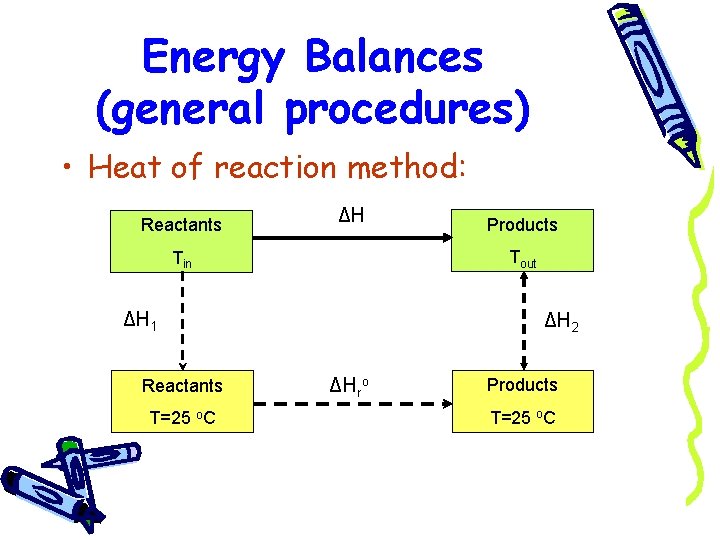
Energy Balances (general procedures) • Heat of reaction method: Reactants ΔH Tout Tin ΔH 1 Reactants T=25 o. C Products ΔH 2 ΔHro Products T=25 o. C
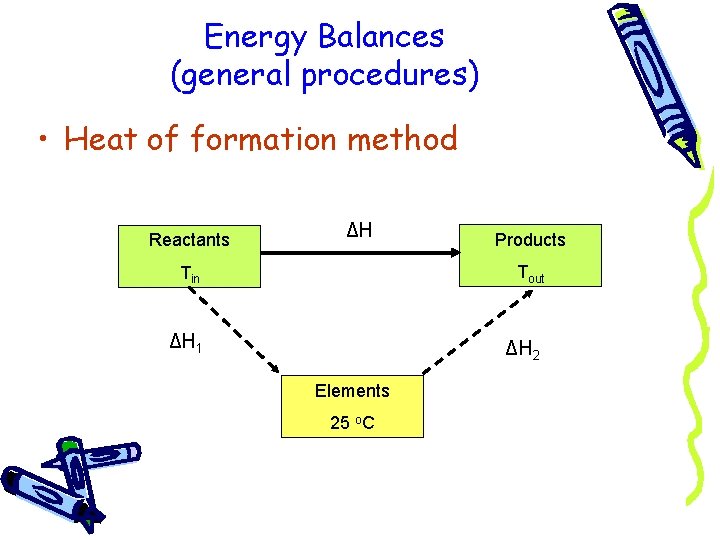
Energy Balances (general procedures) • Heat of formation method Reactants ΔH Products Tin Tout ΔH 1 ΔH 2 Elements 25 o. C
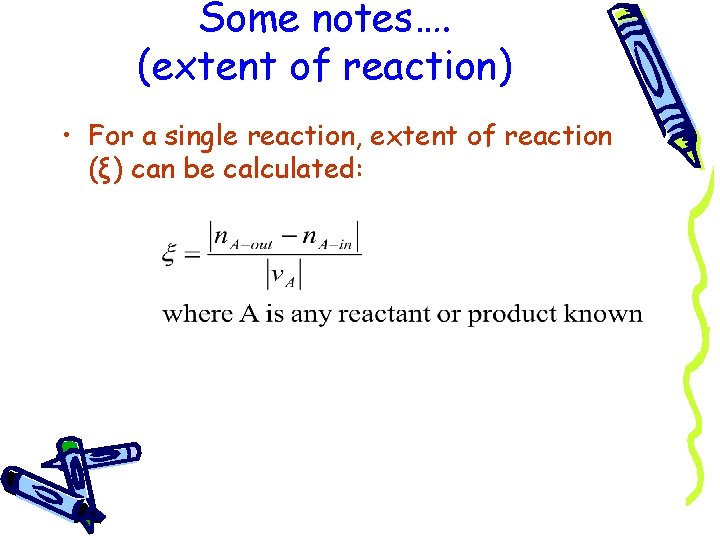
Some notes…. (extent of reaction) • For a single reaction, extent of reaction (ξ) can be calculated:
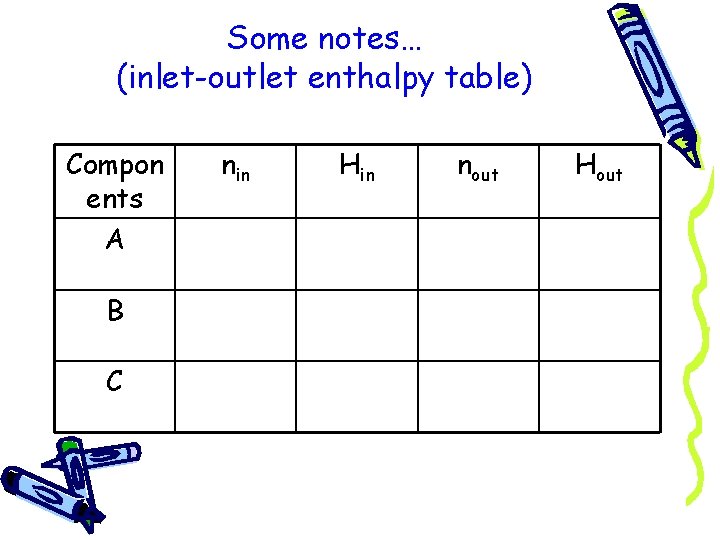
Some notes… (inlet-outlet enthalpy table) Compon ents A B C nin Hin nout Hout

Some notes… • Latent heat transferred without change of T. It could be as heat of vaporization (or condensation). There is a phase change • Sensible heat transferred due to the T difference. There is a change of T, but no phase change Since Cp = f(T), then don’t forget to integrate it. See Table B. 4
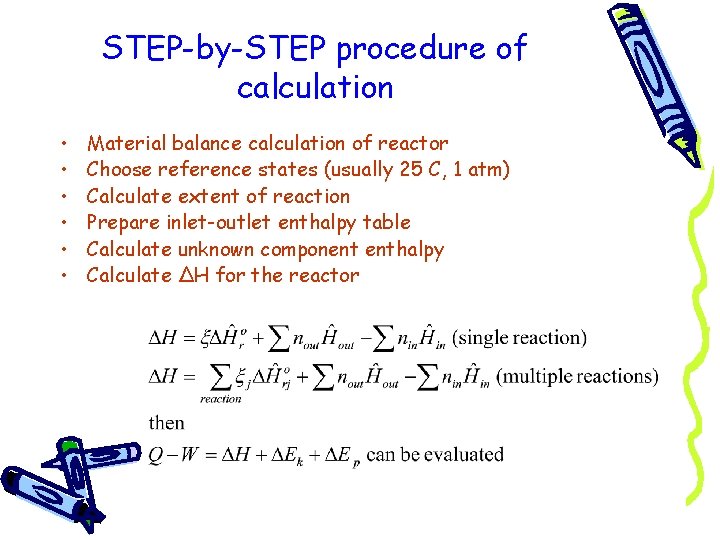
STEP-by-STEP procedure of calculation • • • Material balance calculation of reactor Choose reference states (usually 25 C, 1 atm) Calculate extent of reaction Prepare inlet-outlet enthalpy table Calculate unknown component enthalpy Calculate ΔH for the reactor
Working Session Example 9. 5 -1
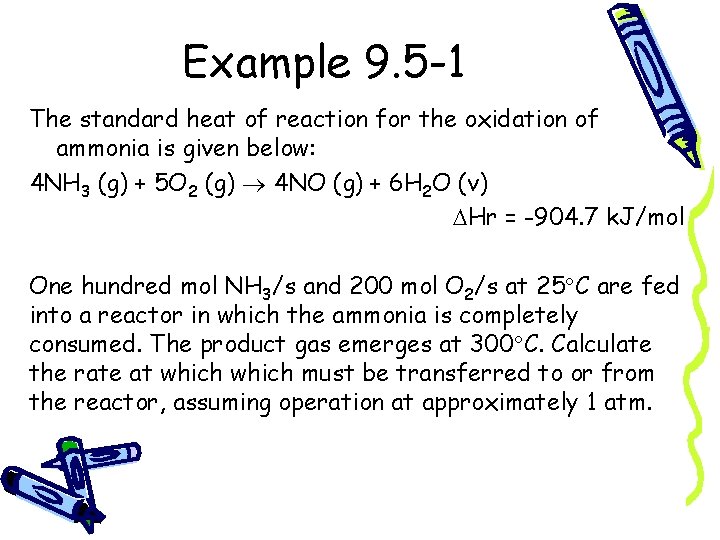
Example 9. 5 -1 The standard heat of reaction for the oxidation of ammonia is given below: 4 NH 3 (g) + 5 O 2 (g) 4 NO (g) + 6 H 2 O (v) Hr = -904. 7 k. J/mol One hundred mol NH 3/s and 200 mol O 2/s at 25 C are fed into a reactor in which the ammonia is completely consumed. The product gas emerges at 300 C. Calculate the rate at which must be transferred to or from the reactor, assuming operation at approximately 1 atm.
Working Session Examples 9. 5 -2 9. 5 -3 9. 5 -4
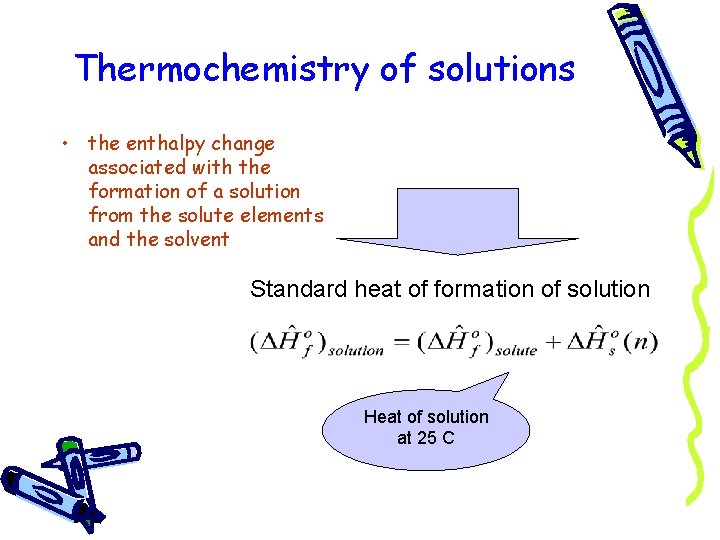
Thermochemistry of solutions • the enthalpy change associated with the formation of a solution from the solute elements and the solvent Standard heat of formation of solution Heat of solution at 25 C
Working Session Example 9. 5 -5
- Slides: 23