ENERGY BALANCE FOR BIOLOGICAL SYSTEM PTT108 Material and

ENERGY BALANCE FOR BIOLOGICAL SYSTEM PTT-108 - Material and Energy Balance By Noor Amirah Abdul Halim
HEAT OF REACTION FOR PROCESSES WITH BIOMASS PRODUCTION Biochemical reactions in cells do not occur in isolation but are linked in a complex array of metabolic transformations. Catabolic and anabolic reactions take place at the same time, so that energy released in one reaction is used in other energy- requiring processes. Cells use chemical energy quite efficiently; however some is inevitably released as heat. How can we estimate the heat of reaction associated with cell metabolism and growth?
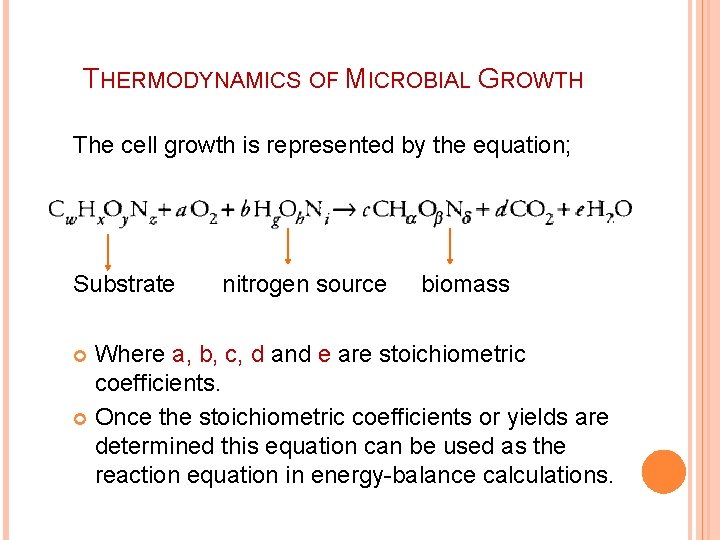
THERMODYNAMICS OF MICROBIAL GROWTH The cell growth is represented by the equation; Substrate nitrogen source biomass Where a, b, c, d and e are stoichiometric coefficients. Once the stoichiometric coefficients or yields are determined this equation can be used as the reaction equation in energy-balance calculations.
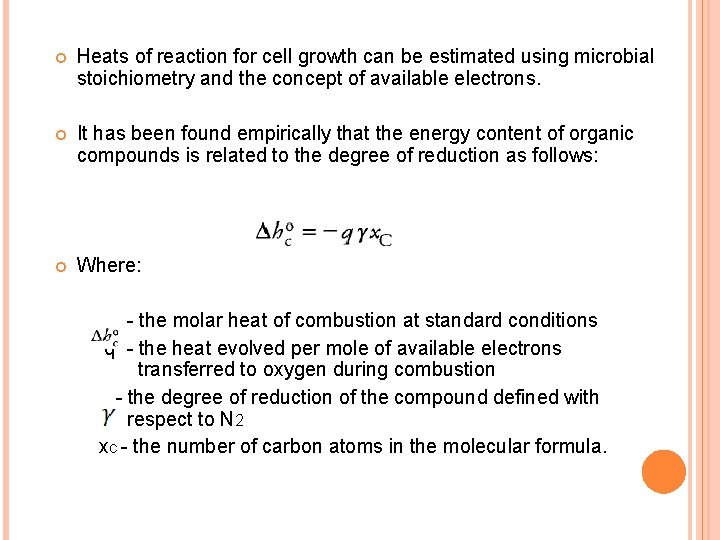
Heats of reaction for cell growth can be estimated using microbial stoichiometry and the concept of available electrons. It has been found empirically that the energy content of organic compounds is related to the degree of reduction as follows: Where: - the molar heat of combustion at standard conditions q - the heat evolved per mole of available electrons transferred to oxygen during combustion - the degree of reduction of the compound defined with respect to N 2 x. C - the number of carbon atoms in the molecular formula.
HEAT OF REACTION WITH OXYGEN AS ELECTRON ACCEPTOR Heat produced in reaction of compounds must be directly proportional to oxygen consumption. Example – Aerobic reaction - In aerobic cultures, we can relate the heat of reaction directly to oxygen consumption, providing a short-cut method for determining the heat of reaction - O 2 is the principal electrons acceptor. If one mole O 2 is consumed during respiration, 4 moles electrons must be transferred. - If the value of 115 k. J energy released per gmol electrons transferred, the amount of energy released from consumption of one gmol O 2 is therefore (4 x 115) k. J, or 460 k. J. - Thus, the heat of reaction for aerobic metabolism is approximately 460 k. J per gmol O 2 consumed. -
HEAT OF REACTION WITH OXYGEN NOT THE ELECTRON ACCEPTOR If the reaction uses electron acceptors other than O 2, heats of combustion must be used to estimate the heat of reaction. Example- Anaerobic reaction - In anaerobic culture, the equation for standard heat reaction is : - n is number of moles and is the standard molar heat of combustion. Heats of combustion for substrate, NH 3 and product are available from tables.

ENERGY-BALANCE EQUATION FOR CELL CULTURE - has a negative sign because its equal to [enthalpy of products -enthalpy of reactants] whereas the energy-balance equation refers to [enthalpy of inlet streams - enthalpy of outlet streams]. - the mass of liquid evaporated - the latent heat of vaporization * This equation is very applicable for fermentation process and not applicable for single-enzyme conversion
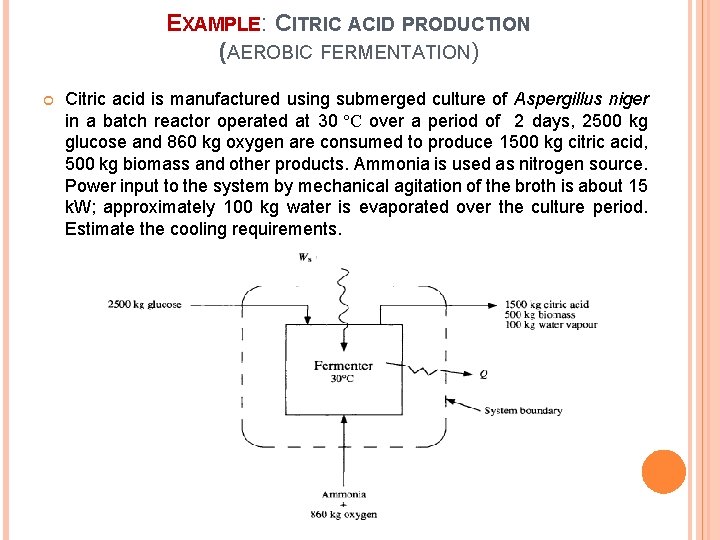
EXAMPLE: CITRIC ACID PRODUCTION (AEROBIC FERMENTATION) Citric acid is manufactured using submerged culture of Aspergillus niger in a batch reactor operated at 30 ºC over a period of 2 days, 2500 kg glucose and 860 kg oxygen are consumed to produce 1500 kg citric acid, 500 kg biomass and other products. Ammonia is used as nitrogen source. Power input to the system by mechanical agitation of the broth is about 15 k. W; approximately 100 kg water is evaporated over the culture period. Estimate the cooling requirements.


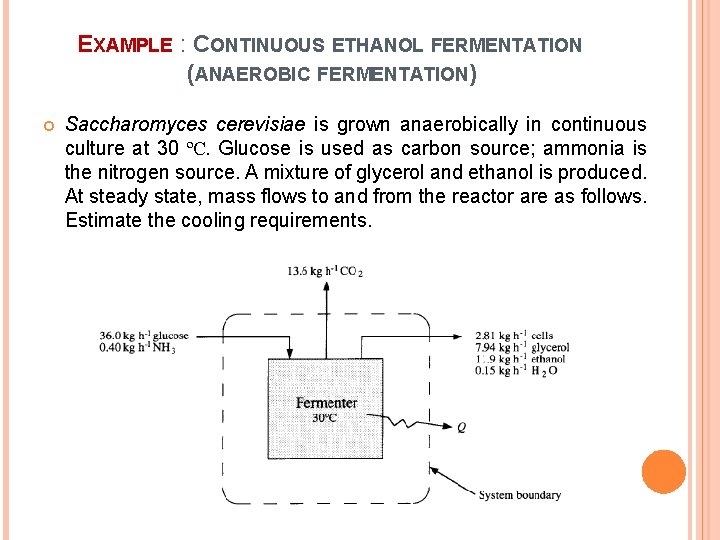
EXAMPLE : CONTINUOUS ETHANOL FERMENTATION (ANAEROBIC FERMENTATION) Saccharomyces cerevisiae is grown anaerobically in continuous culture at 30 ºC. Glucose is used as carbon source; ammonia is the nitrogen source. A mixture of glycerol and ethanol is produced. At steady state, mass flows to and from the reactor are as follows. Estimate the cooling requirements.




THANK YOU
- Slides: 16