Energy and the New Reality Volume 2 CFree















































- Slides: 80

Energy and the New Reality, Volume 2: C-Free Energy Supply Chapter 9: Carbon capture and storage L. D. Danny Harvey harvey@geog. utoronto. ca Publisher: Earthscan, UK Homepage: www. earthscan. co. uk/? tabid=101808 This material is intended for use in lectures, presentations and as handouts to students, and is provided in Powerpoint format so as to allow customization for the individual needs of course instructors. Permission of the author and publisher is required for any other usage. Please see www. earthscan. co. uk for contact details.

Sequence of options: Capture and burial of CO 2 from: • Coal powerplants - why bother? • Natural gas powerplants – if still used to complement wind and solar • Concentrated industrial sources that are hard to displace otherwise • Biomass combustion – to create negative emissions • The atmosphere – after emissions have been eliminated, to begin drawing down atmospheric CO 2 • The atmosphere, and combined with H 2 produced electrolytically from renewable electricity to make synthetic “natural” gas – to use in the existing natural gas distribution network for heating of buildings

Outline • • Sources of CO 2 for capture Capture techniques Energy penalties Compression or liquefaction Disposal sites Environmental, safety and legal issues Timing Strategic considerations

Context: In many scenarios of how fossil fuel CO 2 emissions might be eliminated this century, heavy reliance is placed on shifting transportation and heating to electricity combined with C capture and storage essentially allowing substantial continuation of fossil fuels (along with substantial renewable and nuclear energy and some efficiency improvements in the use of energy to reduce demand)

Definitions: • Carbon Capture and Storage (CCS) refers to the capture and disposal of CO 2 released from industrial processes • This has also been referred to as Carbon Sequestration, but this term has also been applied to the removal of CO 2 from the atmosphere through the buildup of biomass (above-ground vegetation) and/or soil carbon • CCS involving burial of captured CO 2 in geological strata (either on land or under the sea bed), shall be referred to here as geological carbon sequestration, while buildup of soil or plant C shall be referred to as biological carbon sequestration

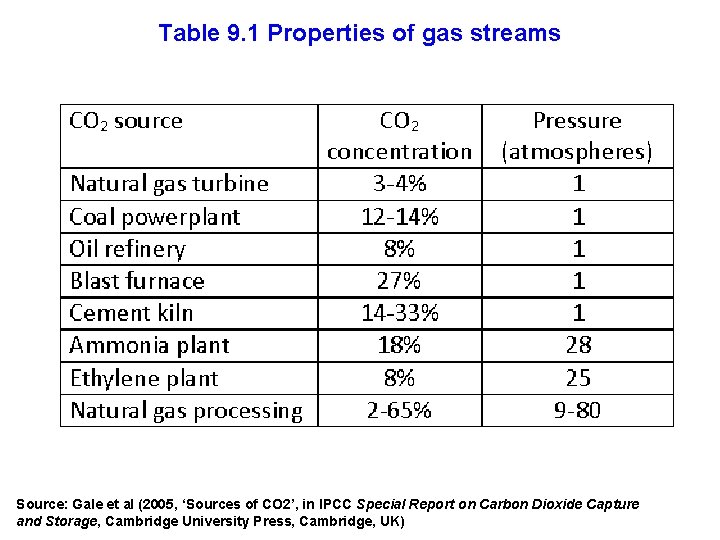
CO 2 is easiest to capture when both the concentration and absolute partial pressure are large

Table 9. 1 Properties of gas streams Source: Gale et al (2005, ‘Sources of CO 2’, in IPCC Special Report on Carbon Dioxide Capture and Storage, Cambridge University Press, Cambridge, UK)

Figure 9. 1 A chemical solvent-based plant that captures a mere 200 t. CO 2/day Source: Thambimuthu et al (2005, IPCC Special Report on Carbon Dioxide Capture and Storage, Cambridge University Press, Cambridge, UK)

All of the stationary CO 2 sources worldwide of 0. 1 Mt. CO 2/yr or more account for about 54% of total world CO 2 emissions (see Table 9. 2)

Options for capture of CO 2 from fossil fuel powerplants: • From the flue gases after normal combustion of fuel in air • From the flue gases after combustion of fuel in pure oxygen (oxyfuel methods in Table 9. 3) • Prior to combustion, during the gasification of coal for IGCC (Integrated gasification-combined cycle) powerplants • During the operation of fuel cells using fossil fuels

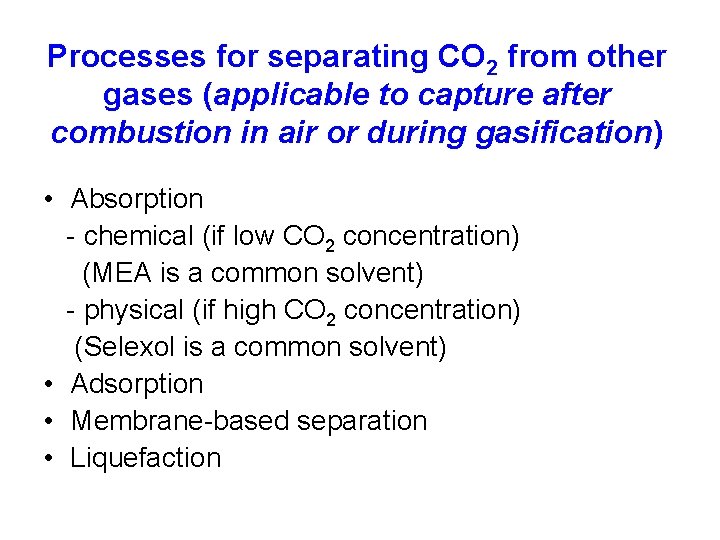
Processes for separating CO 2 from other gases (applicable to capture after combustion in air or during gasification) • Absorption - chemical (if low CO 2 concentration) (MEA is a common solvent) - physical (if high CO 2 concentration) (Selexol is a common solvent) • Adsorption • Membrane-based separation • Liquefaction

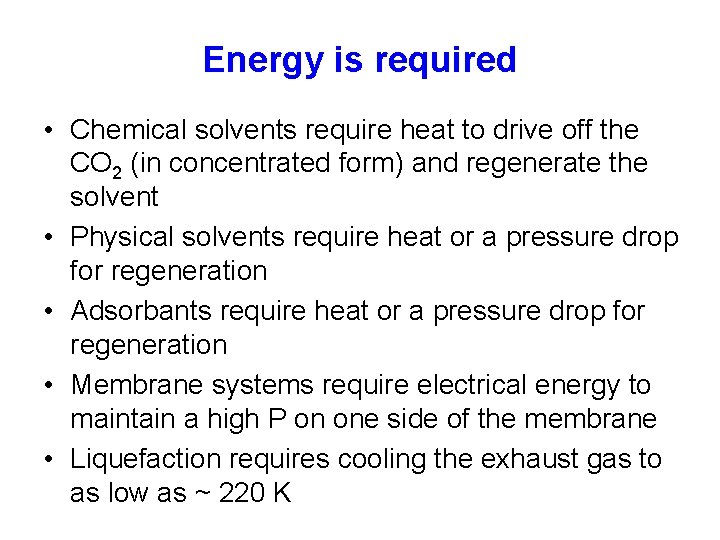
Energy is required • Chemical solvents require heat to drive off the CO 2 (in concentrated form) and regenerate the solvent • Physical solvents require heat or a pressure drop for regeneration • Adsorbants require heat or a pressure drop for regeneration • Membrane systems require electrical energy to maintain a high P on one side of the membrane • Liquefaction requires cooling the exhaust gas to as low as ~ 220 K

Combustion in oxygen • The only gases produced are CO 2 and water vapour • Pure CO 2 is produced by cooling the gas enough to condense out the water vapour (giving 96% CO 2) followed by distillation if desired • Energy is required to separate O 2 from air in liquid form (usually by cooling the air to 89 K, at which point O 2 condenses as a liquid)

IGCC (Integrated Gasification. Combined Cycle) for coal powerplants • Involves converting the coal to CO 2, CO, and H 2 by heating it in 95% oxygen • The CO can be reacted with steam to produce more CO 2 and H 2 • The resulting stream is almost completely CO 2 and H 2, and the CO 2 is easily removed prior to combustion of the H 2 • Conversely, CO and H 2 can be fed to the turbine, burned in air, and the CO 2 removed after combustion using a chemical solvent • Finally, CO and H 2 can be fed to the turbine, burned in pure O 2, and the CO 2 separated by condensing the water vapour that is produced from combustion of the H 2

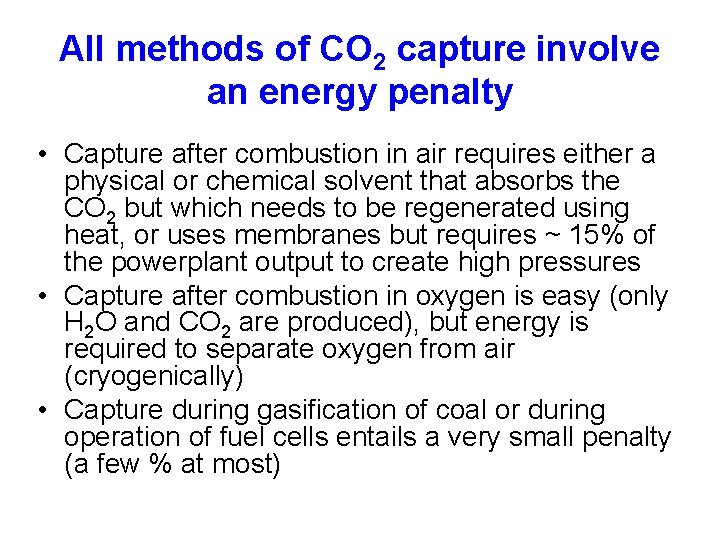
All methods of CO 2 capture involve an energy penalty • Capture after combustion in air requires either a physical or chemical solvent that absorbs the CO 2 but which needs to be regenerated using heat, or uses membranes but requires ~ 15% of the powerplant output to create high pressures • Capture after combustion in oxygen is easy (only H 2 O and CO 2 are produced), but energy is required to separate oxygen from air (cryogenically) • Capture during gasification of coal or during operation of fuel cells entails a very small penalty (a few % at most)

Efficiency Penalties & Costs

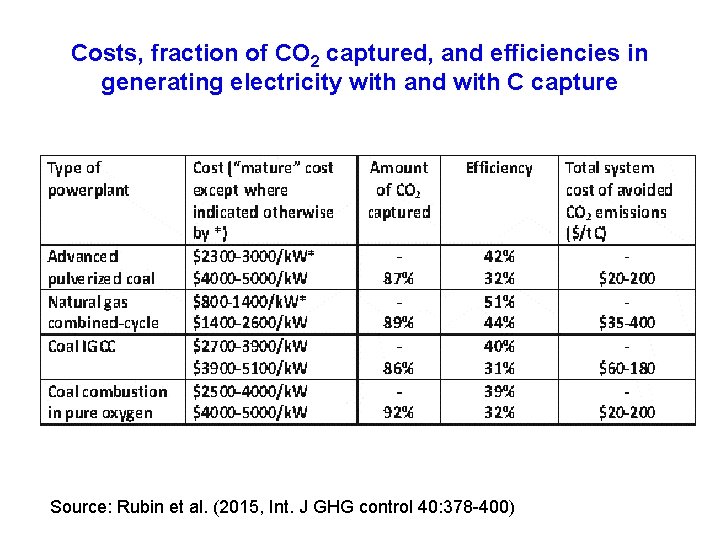
Costs, fraction of CO 2 captured, and efficiencies in generating electricity with and with C capture Source: Rubin et al. (2015, Int. J GHG control 40: 378 -400)

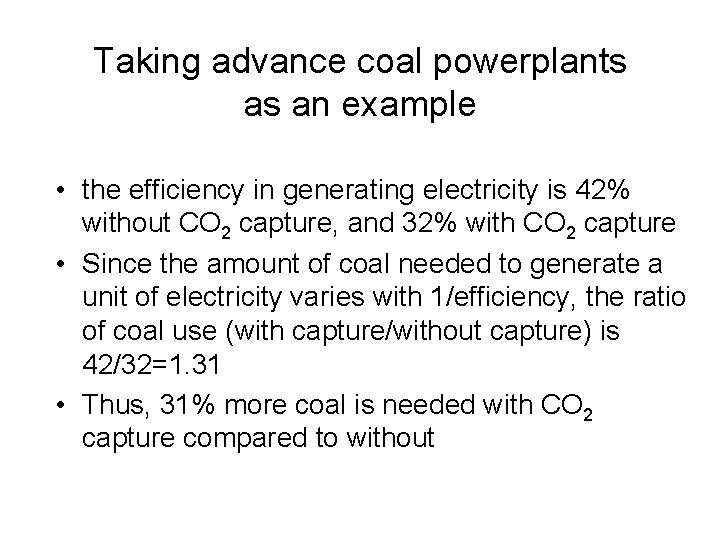
Taking advance coal powerplants as an example • the efficiency in generating electricity is 42% without CO 2 capture, and 32% with CO 2 capture • Since the amount of coal needed to generate a unit of electricity varies with 1/efficiency, the ratio of coal use (with capture/without capture) is 42/32=1. 31 • Thus, 31% more coal is needed with CO 2 capture compared to without

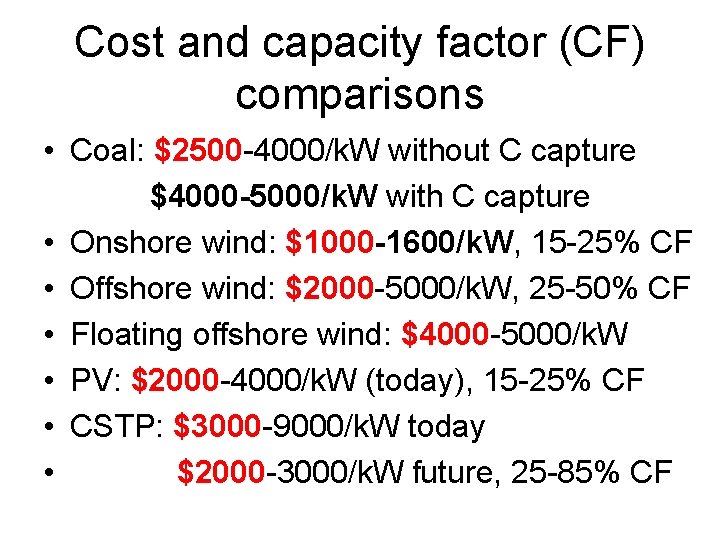
Cost and capacity factor (CF) comparisons • Coal: $2500 -4000/k. W without C capture $4000 -5000/k. W with C capture • Onshore wind: $1000 -1600/k. W, 15 -25% CF • Offshore wind: $2000 -5000/k. W, 25 -50% CF • Floating offshore wind: $4000 -5000/k. W • PV: $2000 -4000/k. W (today), 15 -25% CF • CSTP: $3000 -9000/k. W today • $2000 -3000/k. W future, 25 -85% CF

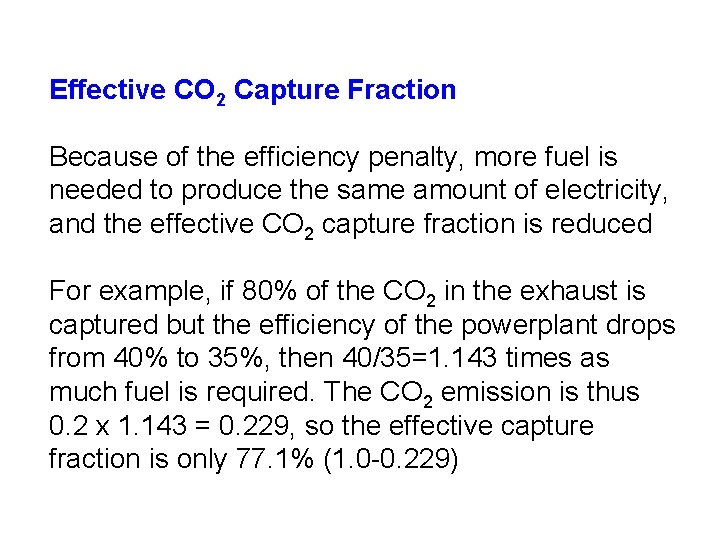
Effective CO 2 Capture Fraction Because of the efficiency penalty, more fuel is needed to produce the same amount of electricity, and the effective CO 2 capture fraction is reduced For example, if 80% of the CO 2 in the exhaust is captured but the efficiency of the powerplant drops from 40% to 35%, then 40/35=1. 143 times as much fuel is required. The CO 2 emission is thus 0. 2 x 1. 143 = 0. 229, so the effective capture fraction is only 77. 1% (1. 0 -0. 229)

Pilot Projects: A proposed 450 -MW IGCC powerplant with carbon capture in Saskatchewan was abandoned after estimated costs ballooned from Cdn$3778/k. W to Cdn$8444/k. W. The US DOE Future. Gen project (a 275 -MW IGCC plant that would co-produce electricity and hydrogen) was cancelled around 2007 after projected costs rose from $3250/k. W to $6500/k. W (Obama re-instated the project).

At COP 24 (the 24 th meeting of the Conference of Parties to the UN Climate Change Convention), in Bonn in November 2017, 23 governments and additional businesses, lead by Canada and the UK, formed the “Powering Past Coalition” and agreed to the following: “Government partners commit to phasing out existing traditional coal power and placing a moratorium on any new traditional coal power stations without operational carbon capture and storage, within our jurisdictions. Business and other non-government partners commit to powering their operations without coal. All partners commit to supporting clean power through their policies (whether public or corporate, as appropriate) and investments and restricting financing for traditional coal power stations without operational carbon capture and storage. ”

The governments involved are: Alberta, Angola, Austria Belgium, British Columbia Canada, Costa Rica Denmark, El Salvador Fiji, Finland, France Italy, Luxembourg Marshall Islands, Mexico Netherlands, New Zealand Niue, Ontario, Oregon Portugal, Québec Switzerland, United Kingdom Vancouver, Washington

Note that the declaration bans coal unless it has CCS – but even without CCS, coal powerplants are now more expensive than onshore wind and PV, and not much less expensive than offshore wind and CSTP. With CCS, coal powerplants are even more expensive. So, effectively, this means an end of coal for electricity among the participating countries. The hope was expressed by the original partners that, by COP 25 (in 2018), 50 partners will have signed on.

Use of biomass with CCS to create negative CO 2 emissions

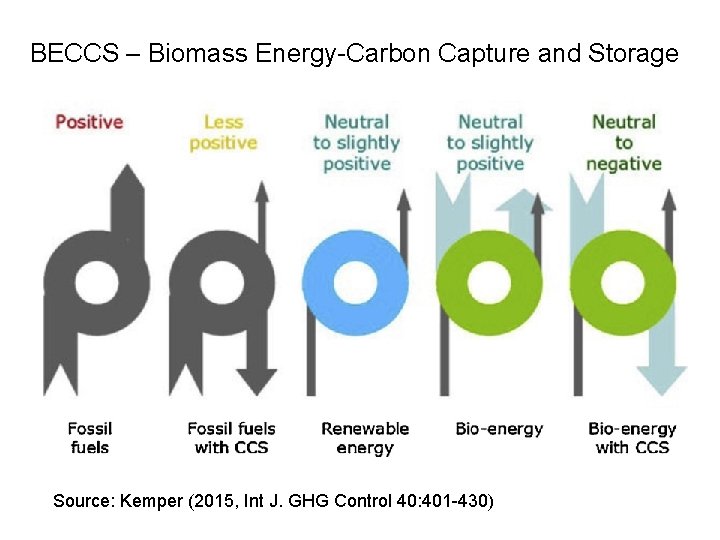
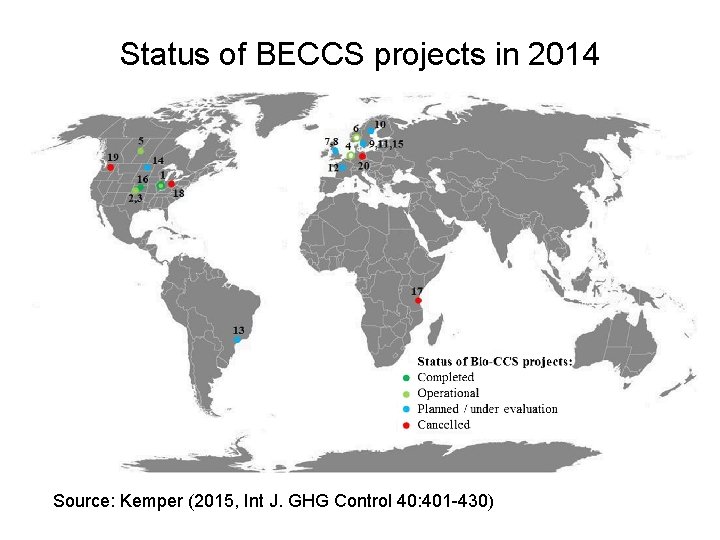
BECCS – Biomass Energy-Carbon Capture and Storage Source: Kemper (2015, Int J. GHG Control 40: 401 -430)

Capturing CO 2 from biomass powerplants The most efficient method of producing electricity from biomass is through biomass integrated gasification combined cycle (BIGCC), a technology that is still under development Gasification of biomass would occur in pure O 2, producing syngas (a mixture of CH 4, CO 2, CO and H 2) and a char residue that is combusted to provide heat for the gasification process.

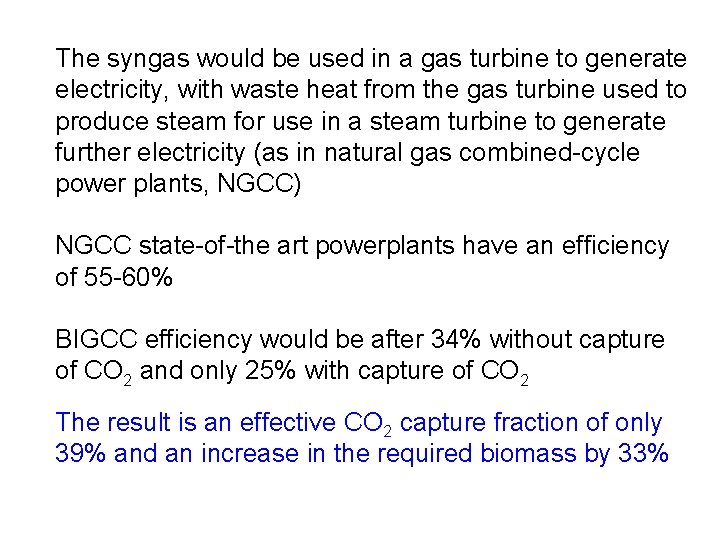
The syngas would be used in a gas turbine to generate electricity, with waste heat from the gas turbine used to produce steam for use in a steam turbine to generate further electricity (as in natural gas combined-cycle power plants, NGCC) NGCC state-of-the art powerplants have an efficiency of 55 -60% BIGCC efficiency would be after 34% without capture of CO 2 and only 25% with capture of CO 2 The result is an effective CO 2 capture fraction of only 39% and an increase in the required biomass by 33%

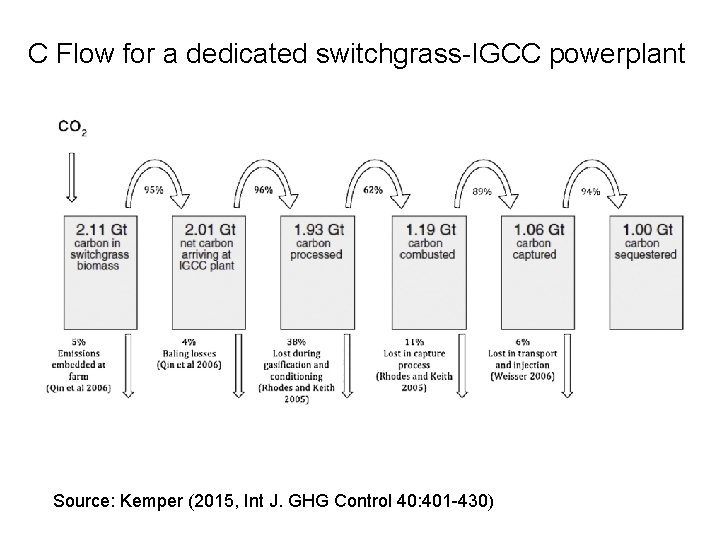
C Flow for a dedicated switchgrass-IGCC powerplant Source: Kemper (2015, Int J. GHG Control 40: 401 -430)

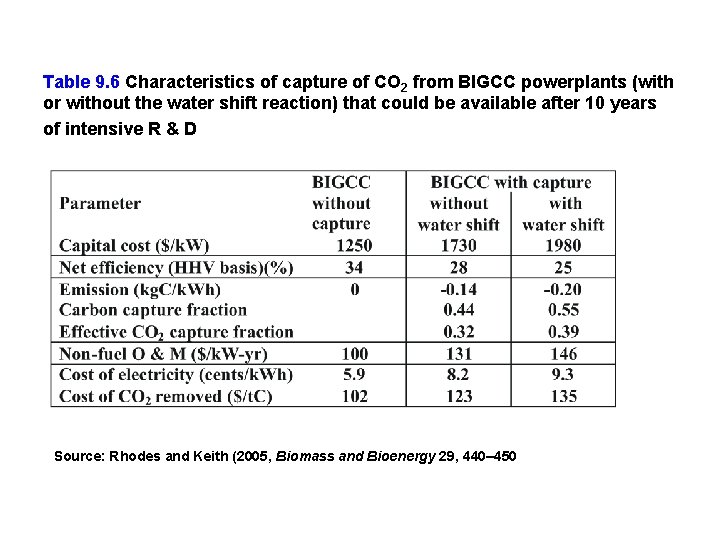
Table 9. 6 Characteristics of capture of CO 2 from BIGCC powerplants (with or without the water shift reaction) that could be available after 10 years of intensive R & D Source: Rhodes and Keith (2005, Biomass and Bioenergy 29, 440– 450

Various schemes for capturing CO 2 that would be produced from gasification of black liquor (a processing waste) in integrated pulp and paper appear to be much more favourable, but would also require many years of intensive research and development

Status of BECCS projects in 2014 Source: Kemper (2015, Int J. GHG Control 40: 401 -430)

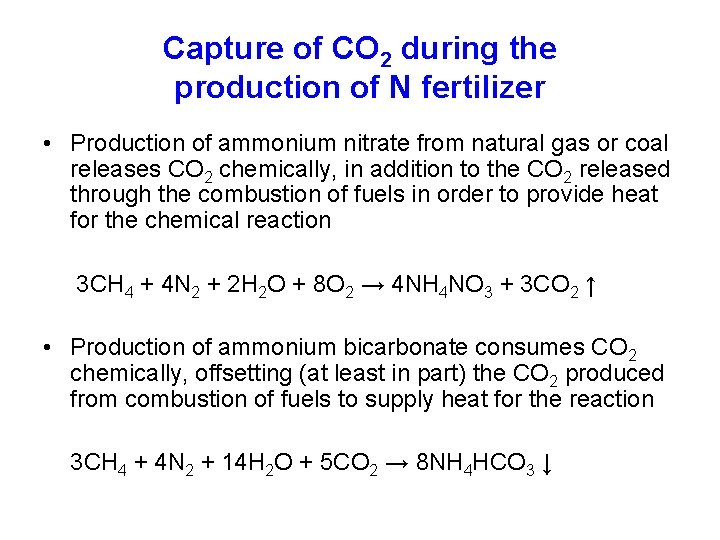
Capture of CO 2 during the production of N fertilizer • Production of ammonium nitrate from natural gas or coal releases CO 2 chemically, in addition to the CO 2 released through the combustion of fuels in order to provide heat for the chemical reaction 3 CH 4 + 4 N 2 + 2 H 2 O + 8 O 2 → 4 NH 4 NO 3 + 3 CO 2 ↑ • Production of ammonium bicarbonate consumes CO 2 chemically, offsetting (at least in part) the CO 2 produced from combustion of fuels to supply heat for the reaction 3 CH 4 + 4 N 2 + 14 H 2 O + 5 CO 2 → 8 NH 4 HCO 3 ↓

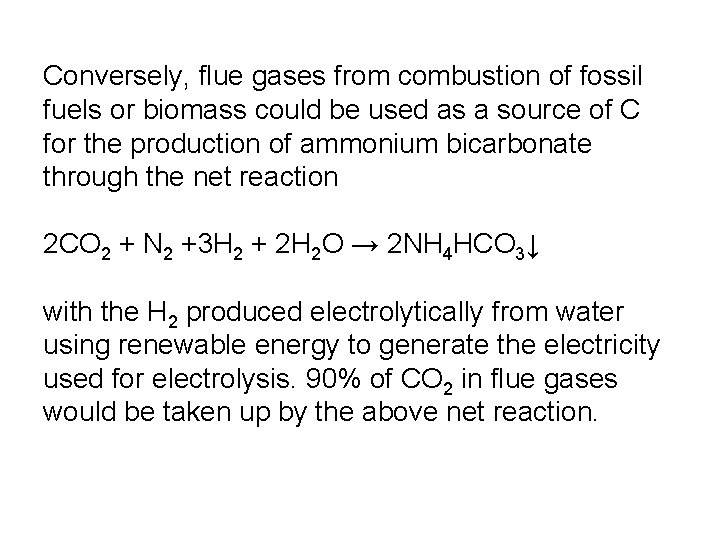
Conversely, flue gases from combustion of fossil fuels or biomass could be used as a source of C for the production of ammonium bicarbonate through the net reaction 2 CO 2 + N 2 +3 H 2 + 2 H 2 O → 2 NH 4 HCO 3↓ with the H 2 produced electrolytically from water using renewable energy to generate the electricity used for electrolysis. 90% of CO 2 in flue gases would be taken up by the above net reaction.

Direct Air Capture (DAC) of CO 2 This entails flow of ambient air over a chemical sorbent that selectively removes CO 2, then releasing the captured CO 2 in concentrated form for collection and disposal, and regeneration of the sorbent

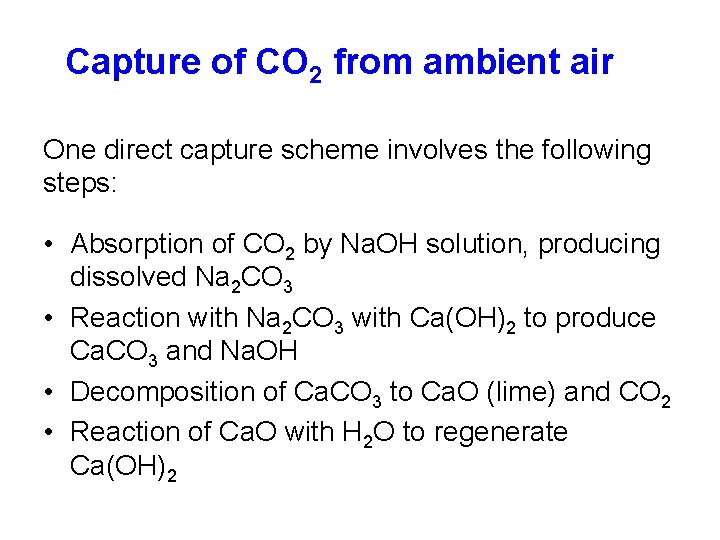
Capture of CO 2 from ambient air One direct capture scheme involves the following steps: • Absorption of CO 2 by Na. OH solution, producing dissolved Na 2 CO 3 • Reaction with Na 2 CO 3 with Ca(OH)2 to produce Ca. CO 3 and Na. OH • Decomposition of Ca. CO 3 to Ca. O (lime) and CO 2 • Reaction of Ca. O with H 2 O to regenerate Ca(OH)2

According to a 2011 assessment of DAC: • The cost is estimated to be $600/t. CO 2, with large uncertainty and higher values more likely than lower values • No demonstration or pilot-scale DAC system had been deployed anywhere • It is entirely possible that no DAC concept under discussion today or yet to be invented will succeed in practice

An enormous physical structure would be required to remove even modest amounts of CO 2 from the atmosphere • To remove CO 2 from the atmosphere at a rate equivalent to the emission from one 1000 -MW (1 -GW) coal power plant would require a structure 10 m high and 30 km long (this would absorb 6 Mt. CO 2/yr). • Lots of electricity would be need to power fans to such air over the absorbing surfaces, and heat would be used to regenerate the absorbing materials • This could be powered by renewable energy, but it would make sense to do this only after all existing electricity emission sources have been replaced with renewable energy sources.

Perspective: • Pre-industrial atmospheric CO 2 concentration was 280 ppmv • The current concentration is 400 ppmv • Under aggressive emission-reduction scenarios, the concentration peaks at 450 -500 ppmv • To draw down the concentration by 50 ppmv means lowering the amount of CO 2 in the atmosphere by roughly 100 Gt • But once we start removing CO 2, the biosphere and oceanic CO 2 sinks get weaker, so to have a sustained lowering by 100 Gt means removing about 200 Gt. C or 733 Gt. CO 2 or 733, 000 Mt CO 2 • If done over a period of 100 years, this means removing about 7300 Mt CO 2/yr – with about 1200 structures 10 m x 30 km.

Compression or liquefaction of captured CO 2

Compression of CO 2 • Compression would be required prior to transport by pipeline, with an energy requirement of 300400 k. Wh/t. C if compressed from 1. 3 to 110 atm • If applied to all of the CO 2 produced by a coal powerplant with 40% efficiency, this corresponds to an energy cost of 7 -10% of the electricity produced (this is in addition to the energy required to capture the CO 2 from the flue gases)

Liquefaction of CO 2 • Liquefaction would be required prior to transport by ship, with an energy requirement of about 400 -440 k. Wh/t. C. • The latter would amount to an efficiency penalty of 10 -12% if applied to the CO 2 produced from a coal powerplant, but less than 2% if applied to the 71% of the CO 2 that can be easily captured while producing H 2 from natural gas

Disposal sites: • Deep saline aquifers on land beneath the ocean bed • Depleted oil and gas fields • Active oil fields, as part of enhanced oil recovery • Coal beds (displacing coal-bed methane) • Injection below the 3000 m depth in the ocean (liquid CO 2 is denser than seawater at this and greater depths)

Storage of CO 2 in deep saline aquifers • Some remains as a gas, under pressure • Some dissolves very slowly into pore water • In aquifers rich in calcium and magnesium silicates, the CO 2 will react with the rock and carbonate will precipitate, reducing the permeability of the rock and creating a permanent trap where none existed before – flood basalts are particularly good

Existing and planned aquifer storage projects: • Sleipner West gas field, underneath the North Sea (off of Norway) • Deep aquifers in Japan and US, planned for Australia, Germany, and Norway

Storage of CO 2 in depleted oil and gas fields and for enhanced oil and gas recovery • CO 2 is currently injected into the base of oil and gas fields in order to increase the oil or gas pressure, thereby increasing the amount of oil or gas that can be extracted • Only the net CO 2 storage should count as credits against emissions • Storage in already-depleted oil and gas fields is another possibility, but would provide no economic credits and, like enhanced oil or gas recovery, would require long-distance transport of CO 2 from the major emission regions to the major oil and gas fields

Storage of CO 2 in coal beds • Coal usually contains methane that is adsorbed onto the surfaces of micro-pores • This methane is call coal-bed methane, and there can be up to 0. 76 GJ methane/tonne of coal (compared to a heating value of coal itself of 32 GJ/t) • CO 2 has a greater affinity for coal, so injection of CO 2 into coal beds will displace methane while being stored in the coal • Up to 2 CO 2 molecules are adsorbed for every CH 4 molecule displaced • The methane would be collected and used as an energy source

Estimated Worldwide Storage Potential on Land • Oil and gas reservoirs: 230 Gt. C • Deep saline aquifers: 55 -15, 000 Gt. C, likely minimum: 270 Gt. C • Coal beds, 16 -54 Gt. C theoretical potential, 2 Gt. C practical potential • TOTAL MINIMUM: about 500 Gt. C, equal to about 100 years of current fossil fuel emissions (about 10 Gt. C/yr) if storing the one half of current fossil fuel emissions that would be amenable to capture

Environmental and Safety Issues Associated with Capture, Transmission and Storage of CO 2

Capture of CO 2 • Emissions during production and transport of materials used for capture of CO 2 (chemical or physical solvents, limestone and ammonia) • Transport and processing of waste produced during regeneration of solvents • Additional requirements for ammonia, limestone and water compared to generation of electricity without CO 2 capture • Increased NOx and ammonia emissions from pulverized-coal powerplants with CO 2 capture

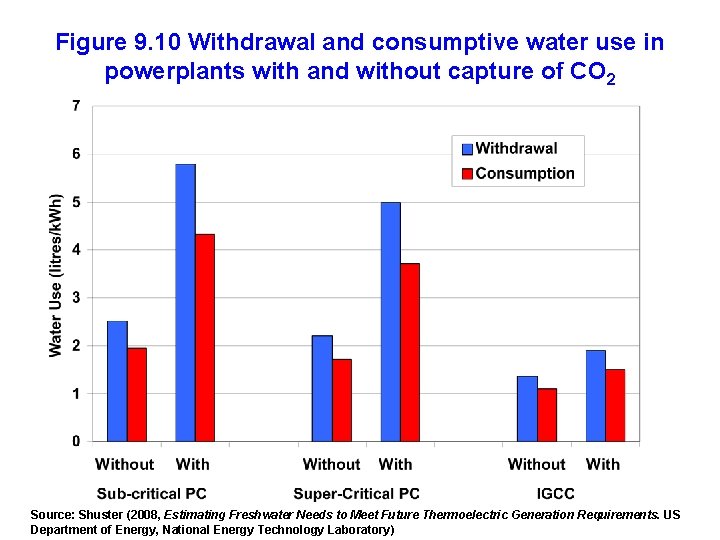
Figure 9. 10 Withdrawal and consumptive water use in powerplants with and without capture of CO 2 Source: Shuster (2008, Estimating Freshwater Needs to Meet Future Thermoelectric Generation Requirements. US Department of Energy, National Energy Technology Laboratory)

Transmission and injection of CO 2 • Rapture of CO 2 pipelines – careful selection of routes needed, constrains locations of capture sites • Well blow-outs during injection of pressurized CO 2 into the ground

Storage in deep saline aquifers • Slow leakage, the potential sources of leakage being: - fracturing of rock during CO 2 injection, which must be carried out at high pressure - leakage through injection and exploration boreholes or through oil-extraction boreholes (of which there are 400, 000 in Alberta and 1 million in Texas, many of which are unmapped) • Upward displacement of saline water into aquifers • Mobilization of toxic materials due to the weak acidity produced as CO 2 reacts with groundwater • Foreclosure of the possibility in the future of using saline groundwater as a source of freshwater (through desalination) or of using minerals dissolved in saline groundwater (Li, Zn and Mn in particular)

Storage in coal beds • A given mass of methane is 72 times as effective as CO 2 in warming the climate over a 25 -year time span, so if only a small fraction of displaced methane leaks to the atmosphere rather than being captured and burnt (for energy), the global warming effect would exceed the benefit from capturing and storing CO 2 • Injection of CO 2 and capture of released CH 4 would require a dense grid of pipes on the ground over the coal beds that are being used

Injection of CO 2 into the deep ocean Note: over a period of about 1000 yrs, about 85% of the CO 2 that humans could ever emit will be absorbed by the oceans, but causing acidification of the oceans at the same time • About 15% of the injected CO 2 eventually leaks into the atmosphere • There would still be global-scale impacts on ocean acidity if 100 s Gt. C were injected • More pronounced acidification would occur near the injection sites

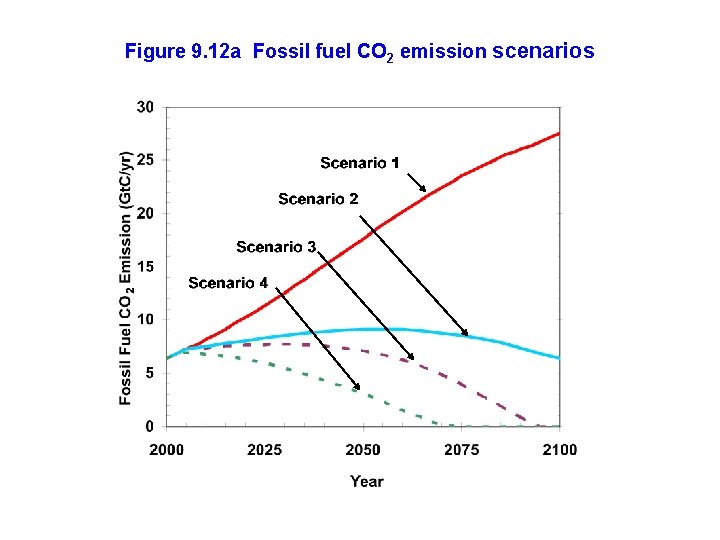
Figure 9. 12 a Fossil fuel CO 2 emission scenarios

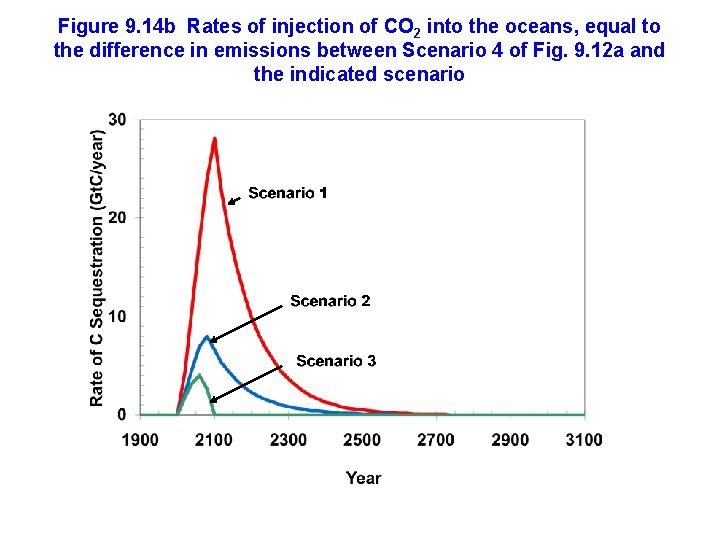
Figure 9. 14 b Rates of injection of CO 2 into the oceans, equal to the difference in emissions between Scenario 4 of Fig. 9. 12 a and the indicated scenario

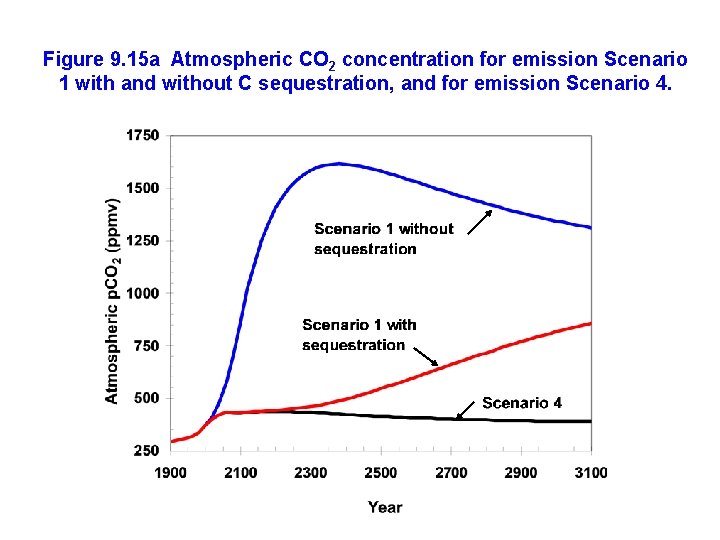
Figure 9. 15 a Atmospheric CO 2 concentration for emission Scenario 1 with and without C sequestration, and for emission Scenario 4.

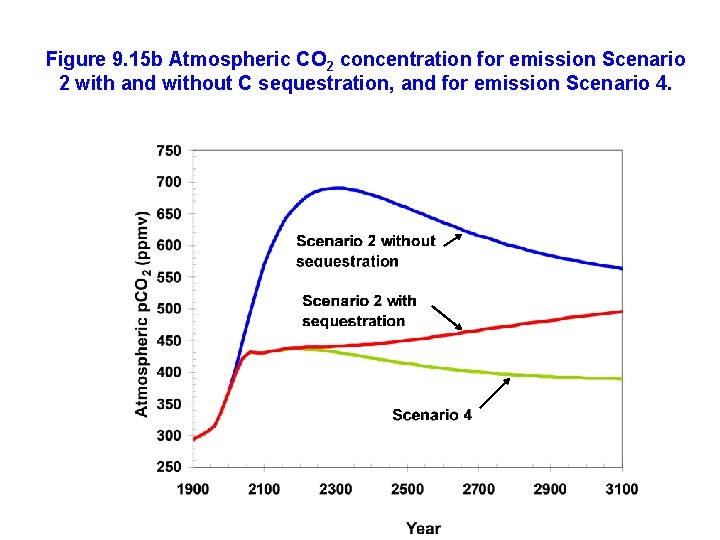
Figure 9. 15 b Atmospheric CO 2 concentration for emission Scenario 2 with and without C sequestration, and for emission Scenario 4.

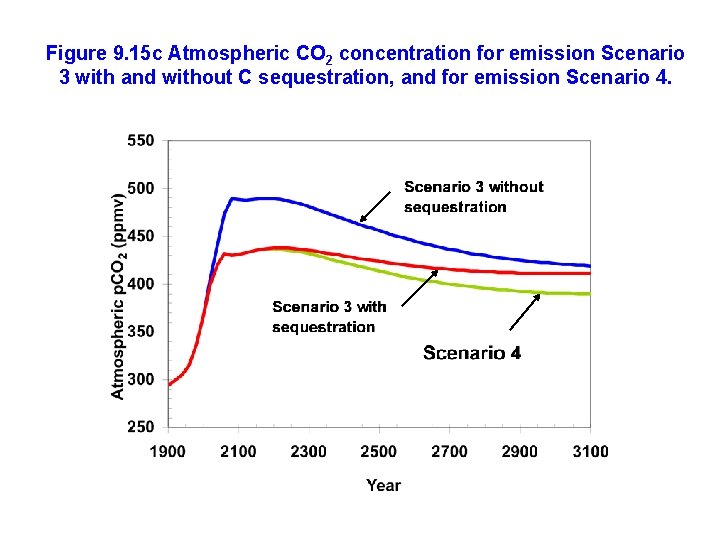
Figure 9. 15 c Atmospheric CO 2 concentration for emission Scenario 3 with and without C sequestration, and for emission Scenario 4.

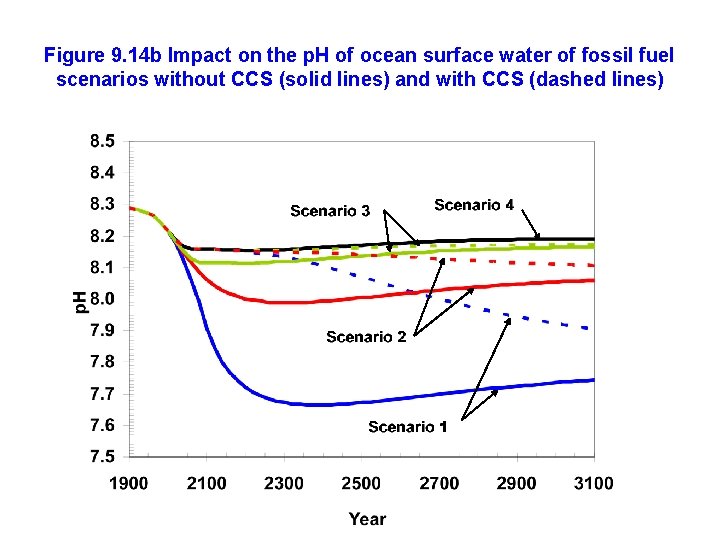
Figure 9. 14 b Impact on the p. H of ocean surface water of fossil fuel scenarios without CCS (solid lines) and with CCS (dashed lines)

Conclusion with regard to injection of CO 2 into deep ocean water: This is environmentally acceptable, if at all, only where CO 2 injection is used as a complement to strong reductions in the use of fossil fuels (accelerating the phase-out of CO 2 emissions from, for example, 2100 to 2070)

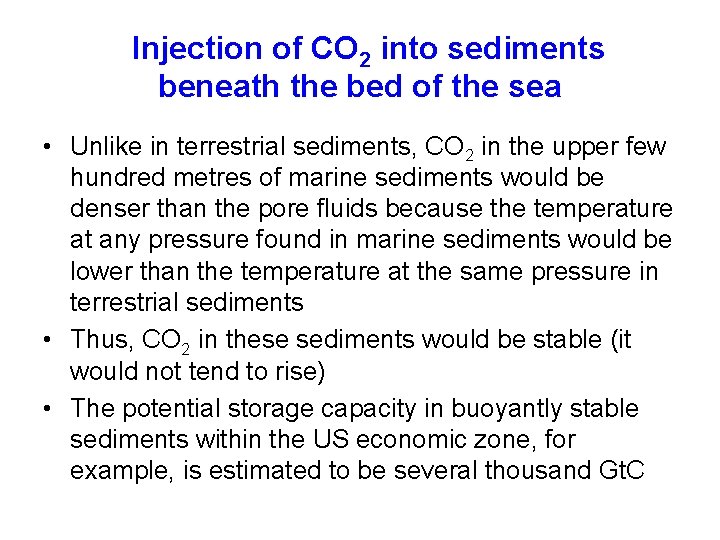
Injection of CO 2 into sediments beneath the bed of the sea • Unlike in terrestrial sediments, CO 2 in the upper few hundred metres of marine sediments would be denser than the pore fluids because the temperature at any pressure found in marine sediments would be lower than the temperature at the same pressure in terrestrial sediments • Thus, CO 2 in these sediments would be stable (it would not tend to rise) • The potential storage capacity in buoyantly stable sediments within the US economic zone, for example, is estimated to be several thousand Gt. C

Legal Issues • A rigorous regulatory process that has broad public and political support will be required if CO 2 is to be sequestered underground on a large scale • Some sort of international monitoring system will be needed if countries or companies are going to engage in international trading of credits related to sequestration of CO 2 • Issues related to prohibition by international conventions of dumping of industrial waste in the ocean will need to be resolved (would fossil fuel CO 2 qualify as an ‘industrial waste’? )

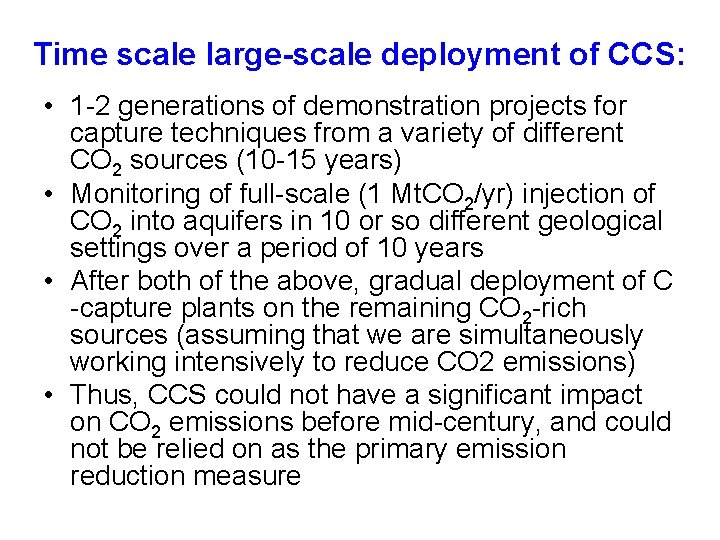
Time scale large-scale deployment of CCS: • 1 -2 generations of demonstration projects for capture techniques from a variety of different CO 2 sources (10 -15 years) • Monitoring of full-scale (1 Mt. CO 2/yr) injection of CO 2 into aquifers in 10 or so different geological settings over a period of 10 years • After both of the above, gradual deployment of C -capture plants on the remaining CO 2 -rich sources (assuming that we are simultaneously working intensively to reduce CO 2 emissions) • Thus, CCS could not have a significant impact on CO 2 emissions before mid-century, and could not be relied on as the primary emission reduction measure

Magnitude • Sequestration of only 1 Gt. C/yr is equivalent to 1/3 of the total current flow of oil out of the ground, with the need for a corresponding infrastructure

Strategic Considerations: • Don’t waste low-cost C storage potential storing CO 2 emissions that can be eliminated in other ways • In particular, don’t waste it on CO 2 from coal plants. We can get coal off of the grid altogether in North America and in Europe in 20 -30 years through a wind/solar/biomass/geothermal/hydro system interconnected with a backbone grid of HVDC lines, almost certainly at less cost than CCS

Strategic Considerations (continued): • Focus on CCS from CO 2 sources that are not so easy to otherwise eliminate – steel, cement, ammonia manufacture in particular • Prepare to deploy CCS of CO 2 released from the use of biomass for electricity generation, so as to create negative CO 2 emissions

Finally, pursuing CCS of coal, even if effective from a climatic point of view, means that the severe landscape impacts associated with coal mining, illustrated in the following slides, will continue.

Alternatives to DAC • We are going to need to actively remove CO 2 from the atmosphere (through “negative” emissions), but DAC does not seem to be even remotely practical on the required scale • The best hope for creating negative emissions seems to be through building up soil C (especially using biochar) and restoring some deforested land (which could be freed up the more diets shift to vegetarian and vegan diets) • There could be a potential to withdraw 1 -2 Gt. C/yr for 100 years or me (100 -200 Gt. C total, enough to lower the concentration by 25 -50 ppmv in the long term)

Some people have considered combining H 2 (produced by electrolysis with C-free electricity) with CO 2 taken from the atmosphere with DAC, to produce synthetic natural gas (SNG) (natural gas is 95 -98% CH 4, with some ethane). This suffers from the same limitation concerning the impracticality of DAC at the required scale. Alternatively, CO 2 captured from any of the sources discussed here could be reacted with H 2 to produce SNG. The SNG would then be used in the existing NG distribution network, which is a major and valuable asset. Thus, we use only the first part of CCS (Carbon capture) and omit the storage part (and all the problems and uncertainties associated with that), and instead use the capture CO 2 to make SNG. Of course, this CO 2 is now ultimately emitted to the atmosphere, but total emission (including from the original CO 2 sources) is reduced

We could have a C-neutral source of SNG if biomass were gasified (producing a mixture of CO and H 2) and then reacted with additional H 2 produced by electrolysis, to produce CH 4. This SNG would be used in place of fossil fuel NG in situations (such as home heating and hot water) where it is too costly to eliminate the use of natural gas [note: I’ve drifted off topic now – making SNG this way is not really CCS]

Some non-climate reasons to phase out the use of coal as quickly as possible

Strip mining in Australia Source: Emily Rochon, Green. Peace

Mountain decapitation in Appalachia Source: Kent Kessinger, www. ilovemountains. org

Coal mine in South Africa Source: Emily Rochon, Green. Peace

Risk of accidents such as this one (collapse of a dam holding coal ash owned by the Tennessee Valley Association in December, 2008) Source: Emily Rochon, Green. Peace

TVA coal dam collapse (continued) Source: Emily Rochon, Green. Peace

TVA coal dam collapse (continued) Source: Emily Rochon, Green. Peace

Tar sands mining in Alberta, Canada, the processing of which is another intensive source of CO 2 that has been considered for CCS while pursuing business-as-usual growth in the rate of extraction. Source: www. petropolis-film. com (Green. Peace)