Energy and Temperature 23215 LO Recall the difference









- Slides: 18

Energy and Temperature 23/2/15 LO: -Recall the difference between energy and temperature -Describe what happens when you heat up solids, liquids & gases - Explain what is meant by equilibrium Starter: Which word is the odd one out for each box? MECHANICAL ENERGY DESTROY FORCE ENERGY MOVE DISSIPATE USEFUL HEAT KINETIC GRAV. POTENTIAL LIGHT Ext: You might hear someone say, “Shut the door cos you’ll let the cold in”. They are wrong. Why?

Starter: Which word is the odd one out for each box? DISSIPATE USEFUL HEAT MECHANICAL ENERGY DESTROY Heat dissipates and is wasted energy in most electrical supplies Energy can’t be destroyed KINETIC GRAV. POTENTIAL LIGHT FORCE ENERGY MOVE Energy doesn’t cause things To move. Forces do Light isn’t to do with movement Ext: You might hear someone say, “Shut the door cos you’ll let the cold in”. They are wrong. Why?

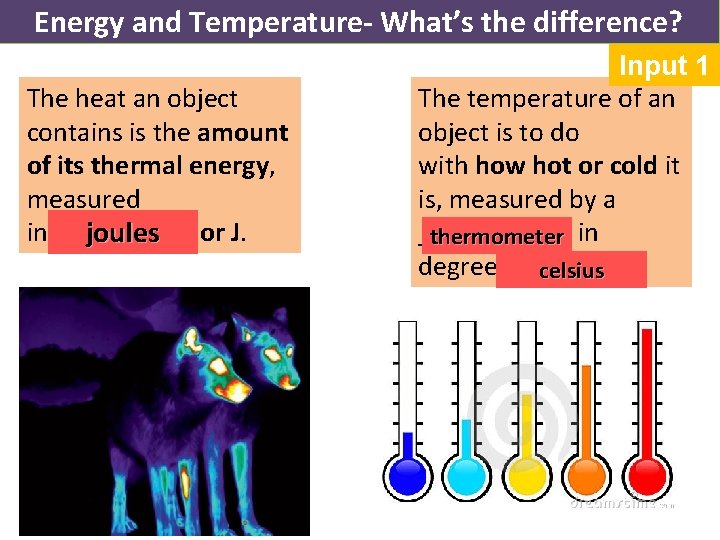
Energy and Temperature- What’s the difference? The heat an object contains is the amount of its thermal energy, measured in _____ joules or J. Input 1 The temperature of an object is to do with how hot or cold it is, measured by a ______ in thermometer degrees ____. celsius

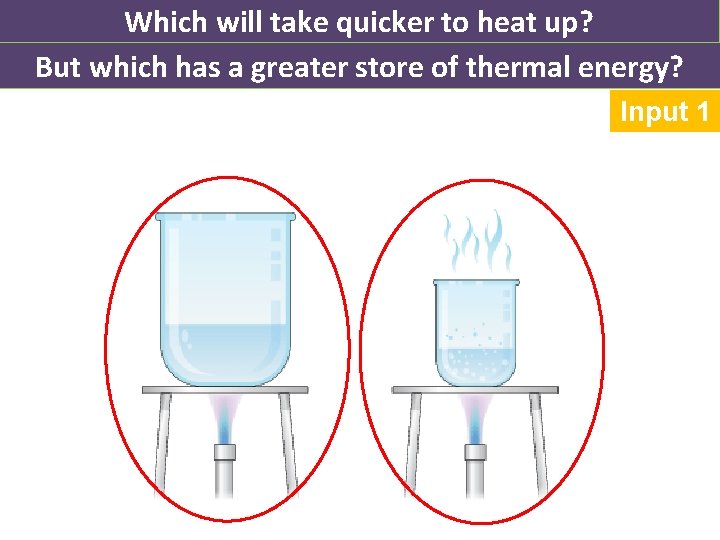
Which will take quicker to heat up? But which has a greater store of thermal energy? Input 1

Swimming pool vs Tea cup Input 1 A swimming pool at 30°C is at a lower temperature than a cup of tea at 80°C. But the swimming pool contains more water, so it stores more thermal energy than the cup of tea. o 30 C o 80 C

Activity 1: Complete this word fill Swimming more temperature thermometer less pool Joules longer slower Celsius You measure ______ in degrees ______ using a ______. Thermal energy is measured in _____. A larger amount of water will take _____ to heat up but will store _____ thermal energy than the smaller object. However, a larger object will lose it’s heat _____ than a smaller object. A cup of tea has ____ thermal energy than a _______

Review Celsius temperature You measure ______ in degrees ______ thermometer using a ______. Thermal energy is measured in _____. A larger amount of water will take Joules more _____ to heat up but will store _____ longer thermal energy than the smaller object. However, a slower larger object will lose it’s heat _____ than a smaller object. A cup of tea has ____ thermal less energy than a _______ Swimming pool

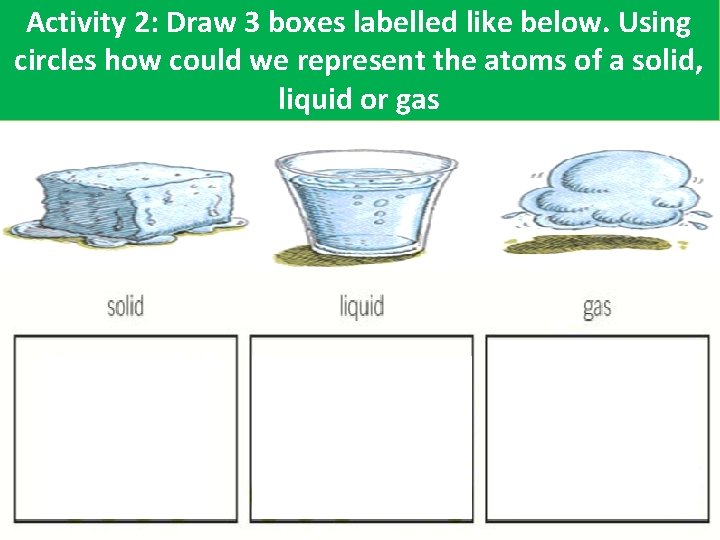
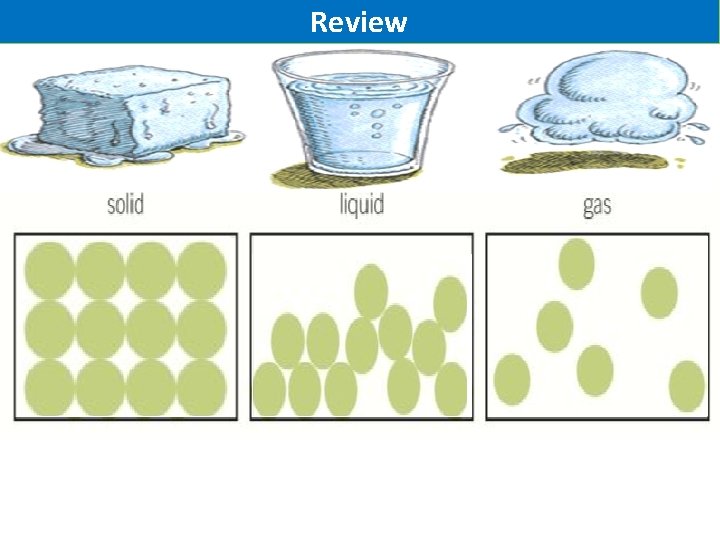
Activity 2: Draw 3 boxes labelled like below. Using circles how could we represent the atoms of a solid, liquid or gas

Review

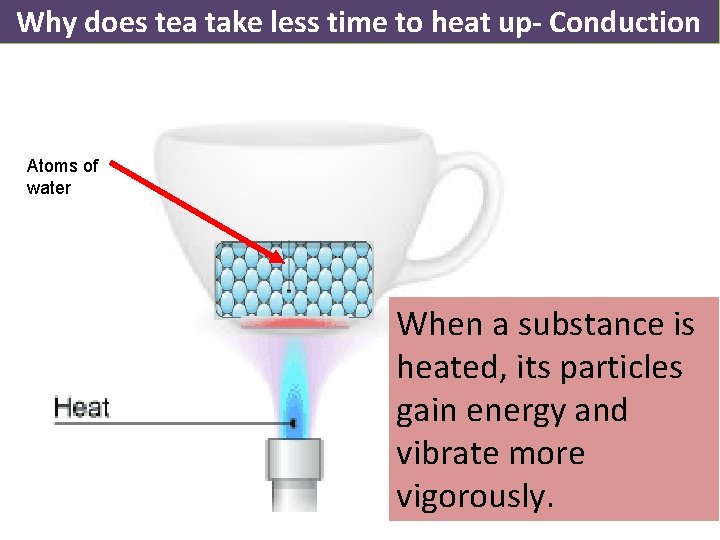
Why does tea take less time to heat up- Conduction Atoms of water When a substance is heated, its particles gain energy and vibrate more vigorously.

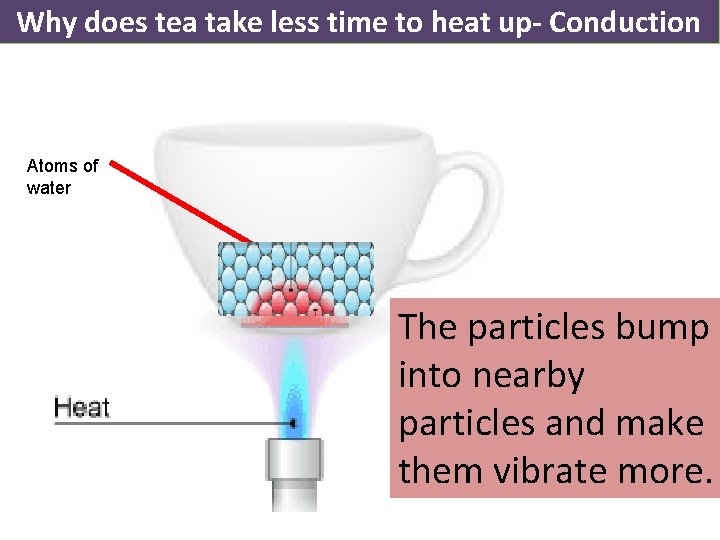
Why does tea take less time to heat up- Conduction Atoms of water The particles bump into nearby particles and make them vibrate more.

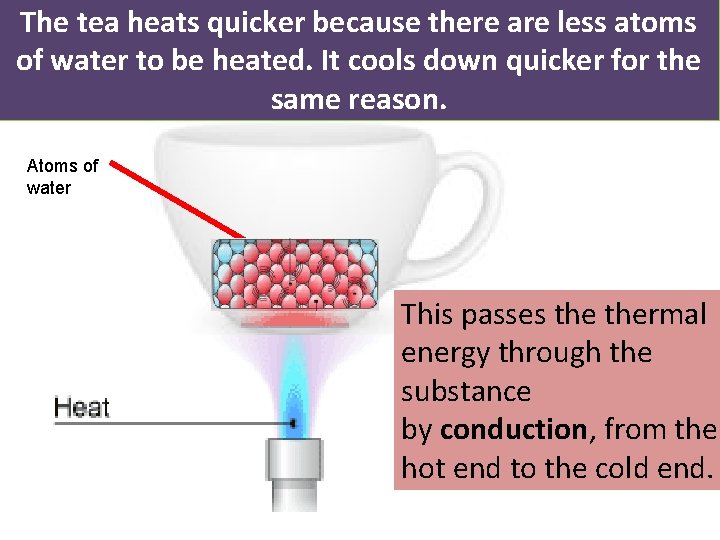
The tea heats quicker because there are less atoms of water to be heated. It cools down quicker for the same reason. Atoms of water This passes thermal energy through the substance by conduction, from the hot end to the cold end.

Joey wants to go for a morning swim. Julia thinks it would be better for him to go for a swim in the sea it will be warmer than the nonheated swimming pool outside. Joey disagrees.

Activity: Use you knowledge of conduction and how the amount of water affects thermal energy and how fast water cools to settle the debate Joey/Julia is right because as the sea/swimming pool Has more atoms it has more T_____ E_______ Over night both the sea and swimming pool will cool down but Because the sea/swimming pool has more atoms it will…

Review Joey/Julia is right because as the sea/swimming pool Has more atoms it has more Thermal Energy Over night both the sea and swimming pool will cool down but because the sea has more atoms it will longer for the heat to DISSIPATES so Joey should go into the sea if he wants to be warmer

Equilibrium means the same (everything equal) Everything likes to be in equilibrium -Water -particles Including air When something isn’t in equilibrium it moves

Thanks for staying. Shut the door behind you! Ext: You might hear someone say, “Shut the door cos you’ll let the cold in”. They are wrong. Why? Cold air doesn’t move in. It is the heat that dissipates into the surroundings making the air inside colder. The longer the door is open the more heat that espaces until equilibrium is reach

Chose a Number One to Five