Energy and Metabolism Chapter 8 Energy Energy Energy

Energy and Metabolism Chapter 8
Energy

Energy

Energy
Metabolism • All the chemical reactions carried out by the cell
Metabolism • Catabolic reactions: • Break down large molecules into smaller substances • Exergonic: • Releases energy
Metabolism • Anabolic reactions: • Synthesis of large molecules from smaller substances • Endergonic: • Requires energy
Metabolism • • Biochemical pathways: Reactions in a cell Occur in sequence Product of one reaction Becomes substrate in the next Pathways are highly regulated & coordinated Feedback inhibition: End product of a reaction blocks the pathway from producing more.
Energy
Energy • Bioenergetics: • Analysis of how energy powers activities of living systems • Growth, order, reproduction, responsiveness & regulation
Energy • • • Energy: The capacity to do work Kinetic energy: Energy of motion Potential energy: Energy of position or stored energy
Energy • Kinetic energy: • Potential energy:
Energy • • Thermodynamics: Study of energy “heat changes” Most work done by living organisms Transformation of PE to KE
Energy • • Sun main source of energy Combines smaller molecules Make larger molecules Energy is stored in the chemical bond
Energy • Redox(oxidation-reduction) reactions: • Transfer of an electron or electrons • Important in the flow of energy in biological systems • An electron is passed from one atom to another energy is passed
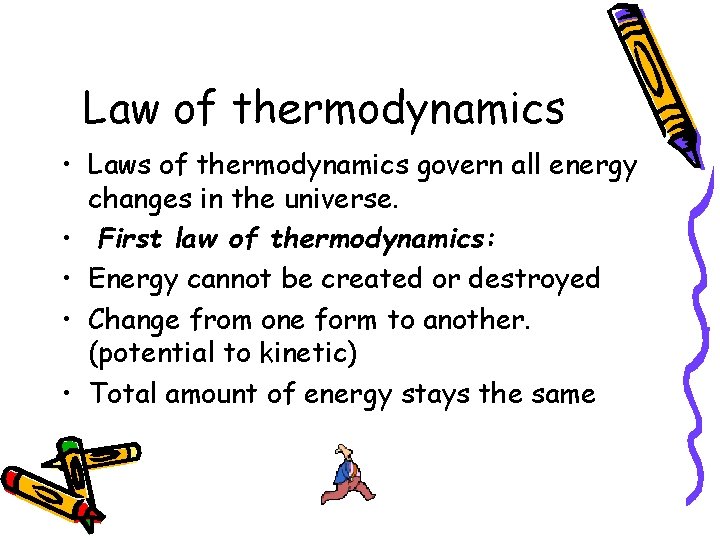
Law of thermodynamics • Laws of thermodynamics govern all energy changes in the universe. • First law of thermodynamics: • Energy cannot be created or destroyed • Change from one form to another. (potential to kinetic) • Total amount of energy stays the same

First law • In living organisms: • Eating transfers energy from the bonds in food to organism • PE is transferred to KE
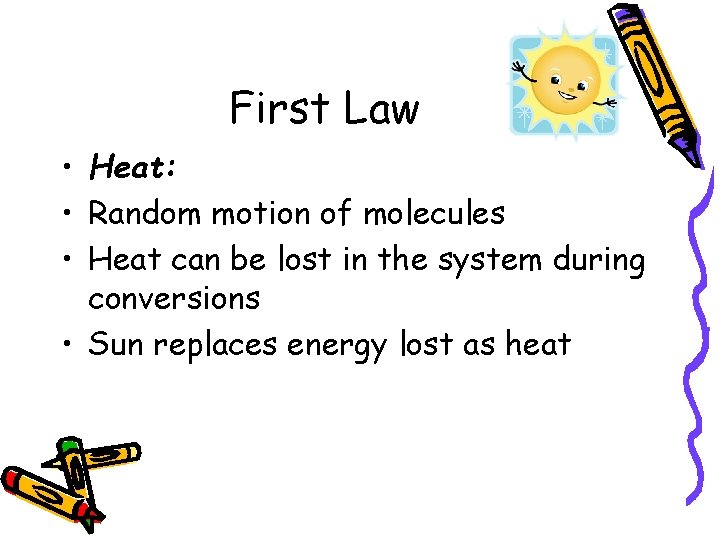
First Law • Heat: • Random motion of molecules • Heat can be lost in the system during conversions • Sun replaces energy lost as heat
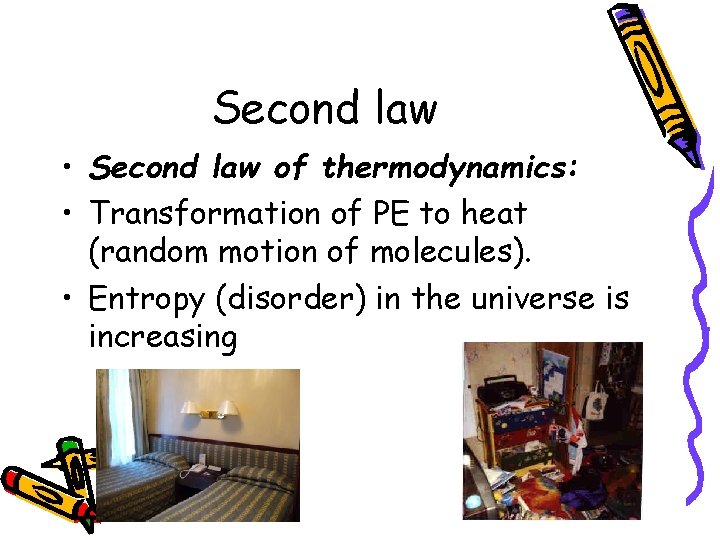
Second law • Second law of thermodynamics: • Transformation of PE to heat (random motion of molecules). • Entropy (disorder) in the universe is increasing
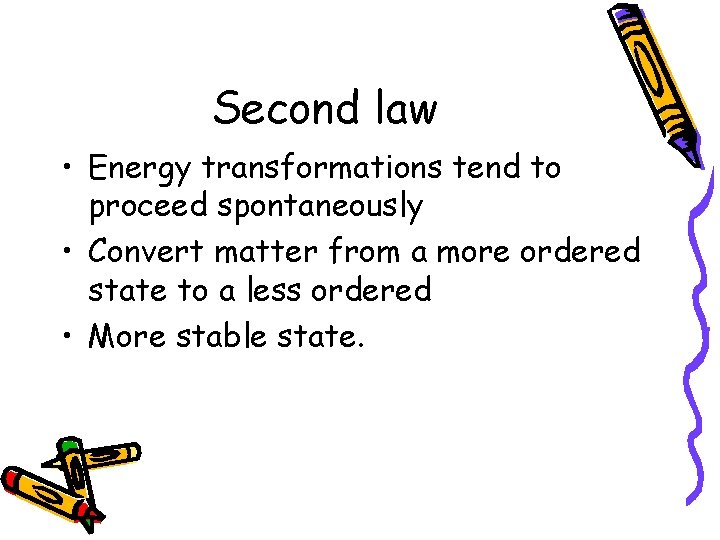
Second law • Energy transformations tend to proceed spontaneously • Convert matter from a more ordered state to a less ordered • More stable state.

Second law • • • Entropy(s): Disorder in a system Enthalpy (H): Heat content Free energy(G): Amount of energy available to do work in any system. • Amount of energy available to break and then make other chemical bonds

Second law • • • G=Gibbs free energy G = H - T S (T=Kelvin temp) G is positive Products have more energy than reactants More energy in the bonds or less randomness • Endergonic reaction
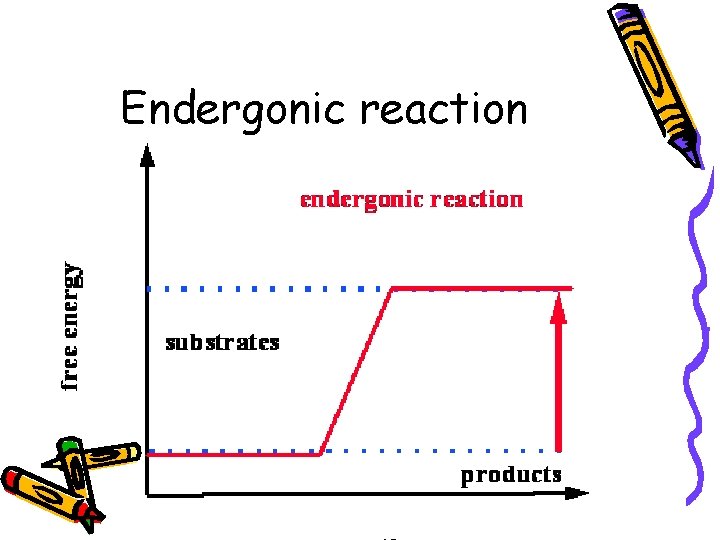
Endergonic reaction

Second law • G is negative • Products have less energy than reactants • H is lower (bond energy) or S is greater- more randomness • Exergonic: • Reaction that releases energy
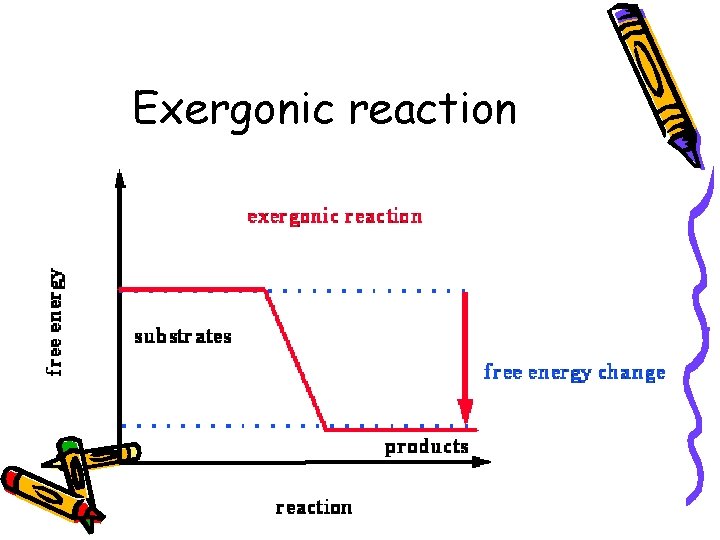
Exergonic reaction
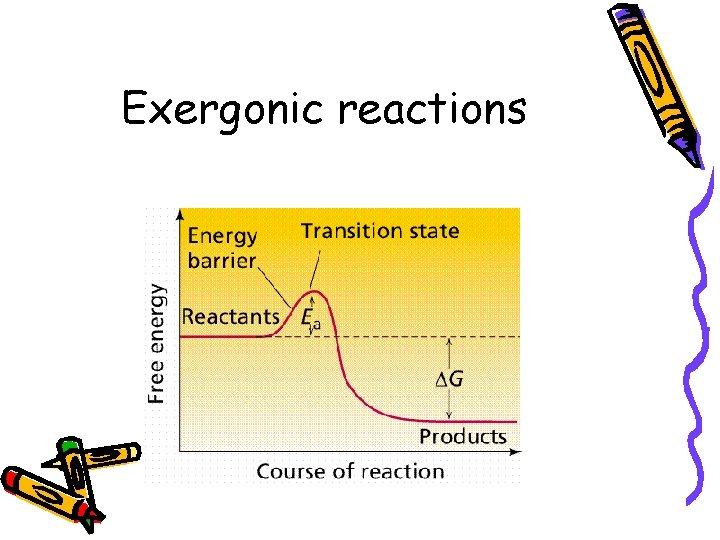
Exergonic reactions

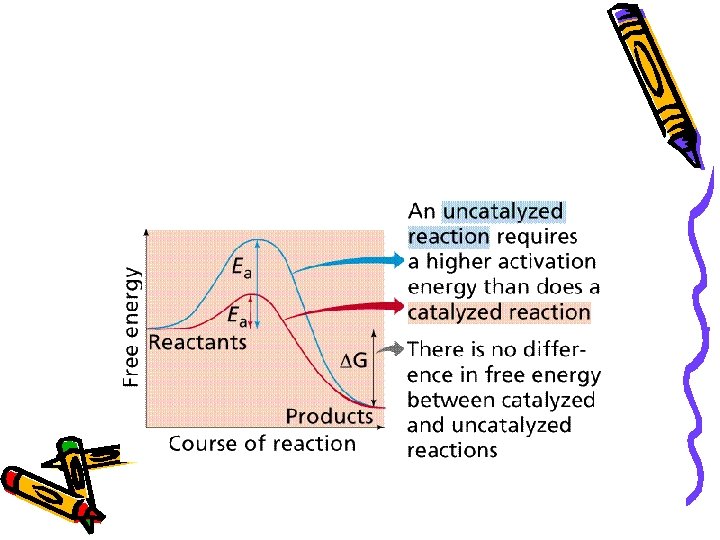
Activation Energy • Energy needed to initiate a reaction • All reactions require activation energy. • Reactions with higher AE tend to move forward more slowly
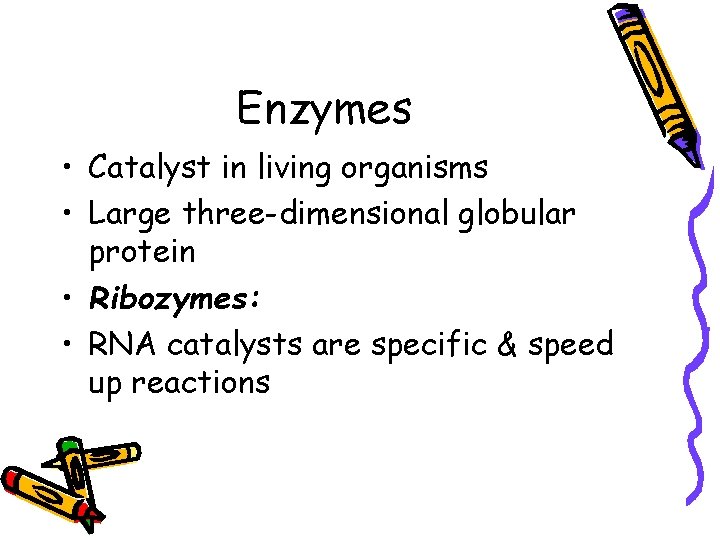
Enzymes • Catalyst in living organisms • Large three-dimensional globular protein • Ribozymes: • RNA catalysts are specific & speed up reactions

Enzymes • • • Substrate: Molecule that is going to undergo the reaction Active sites: Specific spots on the enzyme that substrates binds Enzyme-substrate complex: Enzymes bind to substrates with a precise fit. Induced fit: Substrate causes the enzyme to adjust to make a better fit E+S ES E + P
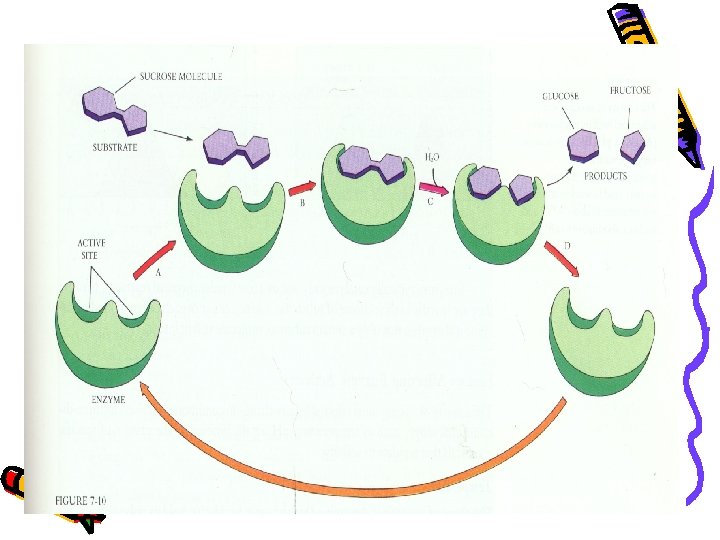
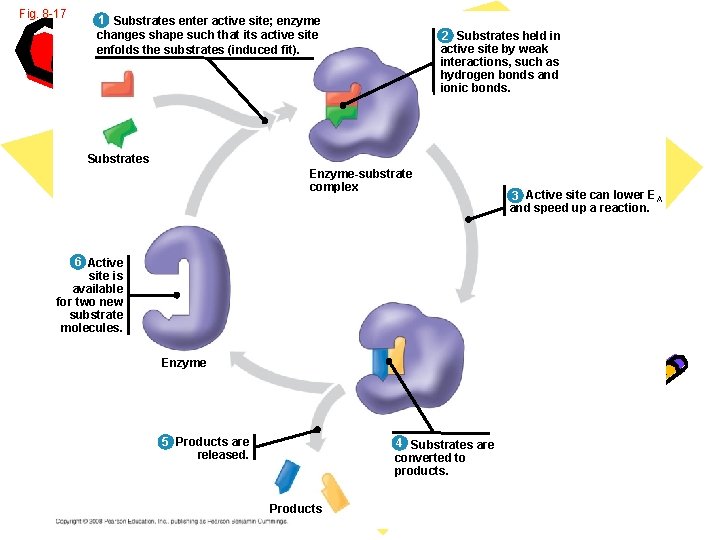
Fig. 8 -17 1 Substrates enter active site; enzyme changes shape such that its active site enfolds the substrates (induced fit). 2 Substrates held in active site by weak interactions, such as hydrogen bonds and ionic bonds. Substrates Enzyme-substrate complex 6 Active site is available for two new substrate molecules. Enzyme 5 Products are released. 4 Substrates are converted to products. Products 3 Active site can lower EA and speed up a reaction.

Enzymes • • • Only small amounts are necessary Can be recycled Specific Speeds up the reactions Different types of cells have different enzymes • Determines course of chemical reactions in the cell
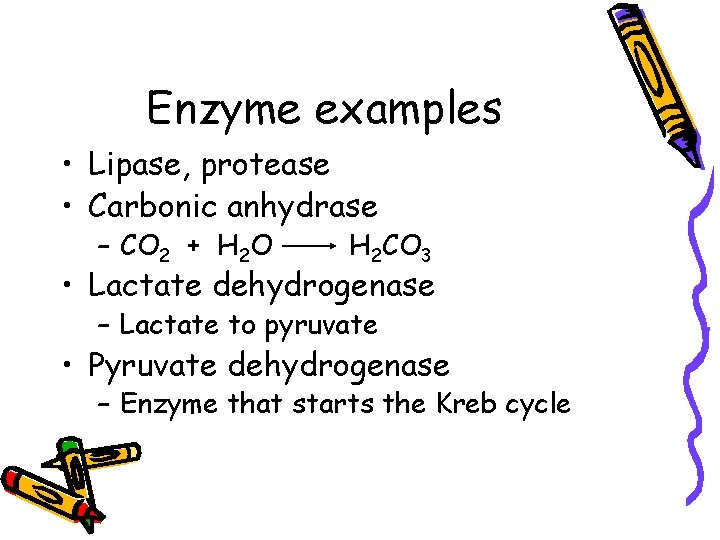
Enzyme examples • Lipase, protease • Carbonic anhydrase – CO 2 + H 2 O H 2 CO 3 • Lactate dehydrogenase – Lactate to pyruvate • Pyruvate dehydrogenase – Enzyme that starts the Kreb cycle
Enzymes • Factors that affect the rate of enzyme • 1. Concentration of enzyme & substrate • 2. Factors that affect 3 -D shape of the enzyme • Temperature, p. H, salt concentration and regulatory molecules

Enzymes • • Inhibitor: Binds the enzyme Prevents it from working Occurs at end of a pathway to stop reactions • Two types of inhibitors • Competitive • Noncompetitive
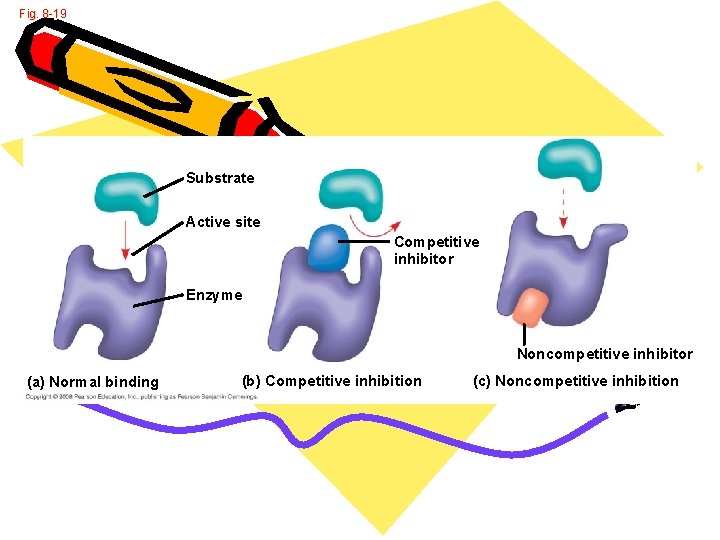
Fig. 8 -19 Substrate Active site Competitive inhibitor Enzyme Noncompetitive inhibitor (a) Normal binding (b) Competitive inhibition (c) Noncompetitive inhibition

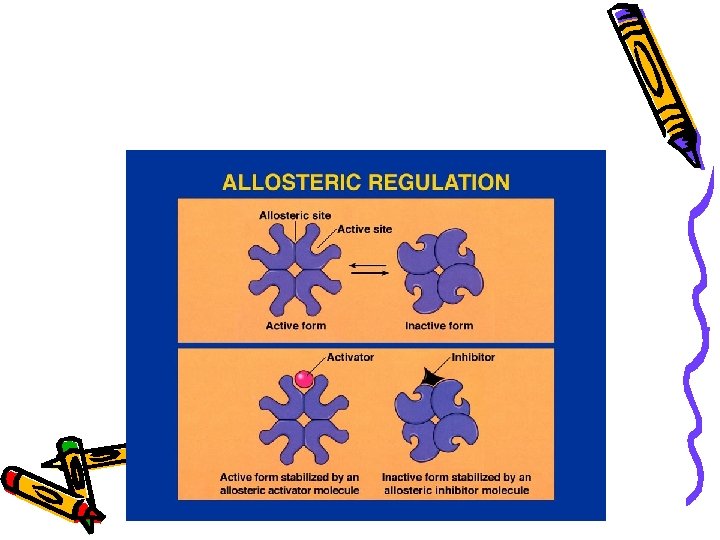
Enzymes • • Allosteric site: On/off switch for the enzyme Usually at different location than the active site Allosteric inhibitor: Binds at the allosteric site Stops the enzyme activity Activators: Binds & increases the activity
Enzymes • • • Cofactor: Assists enzyme function such as Zn, Mg, Cu Coenzymes: Organic molecules that are not proteins Help transfer electrons & energy associated with the electrons • Vitamins are coenzymes • NAD+ important coenzyme

Energy
ATP • ATP powers the energy requiring processes in the cell • 1. Chemical work (making polymers) • 2. Transporting substances • 3. Mechanical work • Muscle movement, cilia
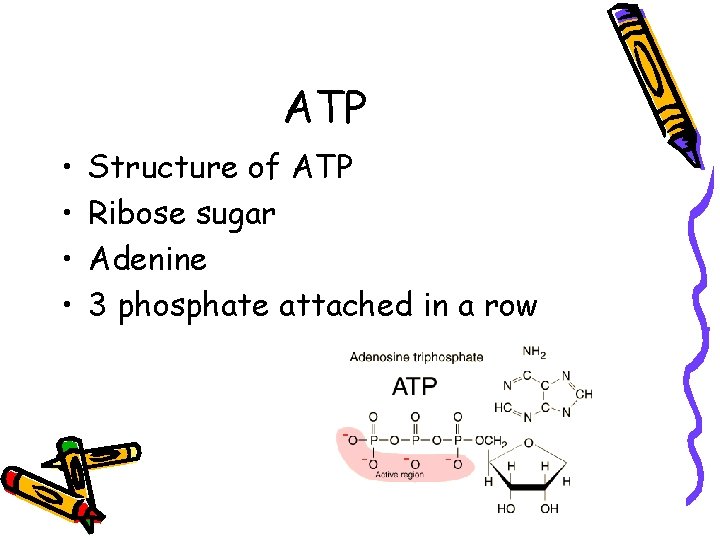
ATP • • Structure of ATP Ribose sugar Adenine 3 phosphate attached in a row
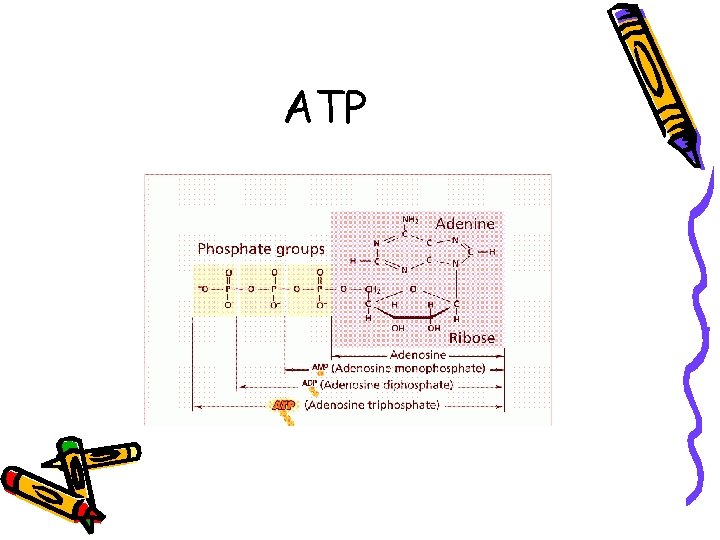
ATP
ATP • • • ATP ADP Losses a inorganic phosphate Hydrolysis 7. 3 kcal/mole of energy is released.
- Slides: 45