Energy and Matter 1 Energy is the capacity








- Slides: 20

Energy and Matter 1

Energy is the capacity to do work or produce heat. n n n Three forms of energy: Radiant, Kinetic, & Potential Law of Conservation of Energy: Energy is neither created or destroyed, just changed from form to form. Measuring energy: n Use a device called a calorimeter to measure heat. n calorie: amount of heat needed to raise 1 g of water 1 °C n 1 Calorie = 1, 000 calories n Joule = SI unit of measure for Energy n 1 cal = 4. 184 J 2

1. Practice with energy conversions Convert 686, 000 Joule to Calories 686, 000 J 163. 9 Cal 1 cal 1 Cal 4. 184 J 1, 000 cal ~164 Cal 2. Convert 47, 500 calorie to Joule 47, 500 cal 4. 184 J 1 cal 198, 740 J ~199, 000 J 3

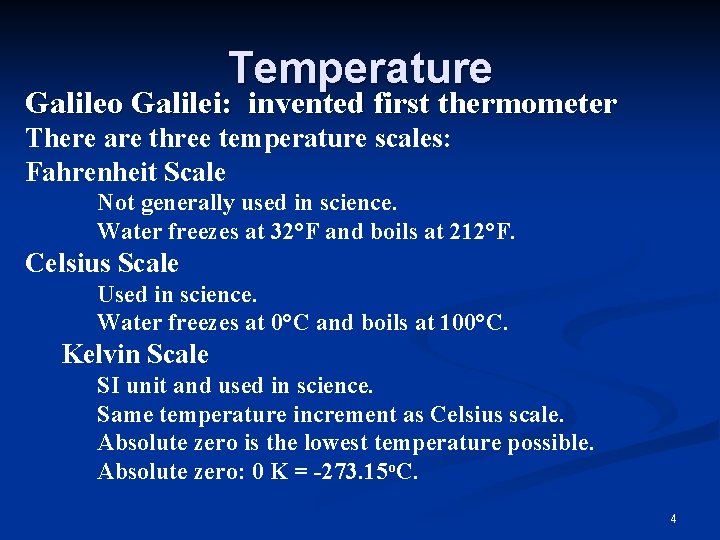
Temperature Galileo Galilei: invented first thermometer There are three temperature scales: Fahrenheit Scale Not generally used in science. Water freezes at 32°F and boils at 212°F. Celsius Scale Used in science. Water freezes at 0°C and boils at 100°C. Kelvin Scale SI unit and used in science. Same temperature increment as Celsius scale. Absolute zero is the lowest temperature possible. Absolute zero: 0 K = -273. 15 o. C. 4

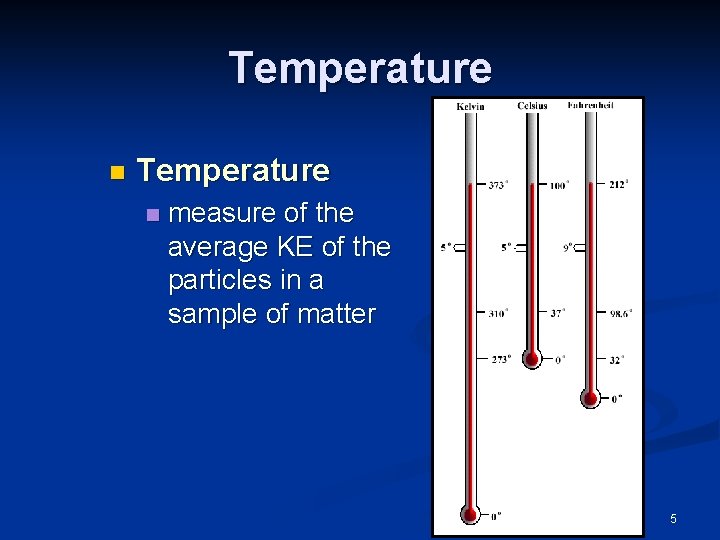
Temperature n measure of the average KE of the particles in a sample of matter 5

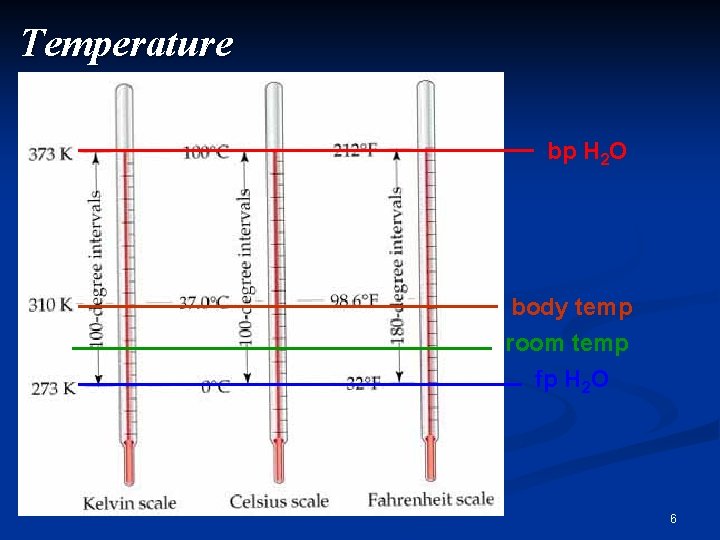
Temperature bp H 2 O body temp room temp fp H 2 O 6

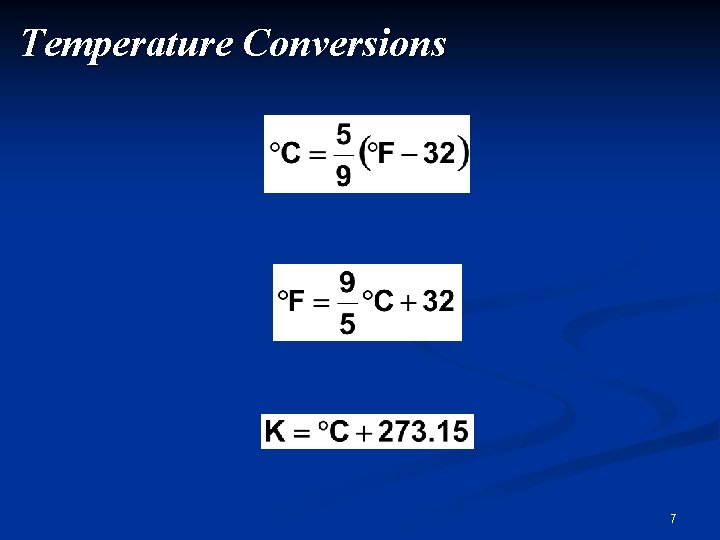
Temperature Conversions 7

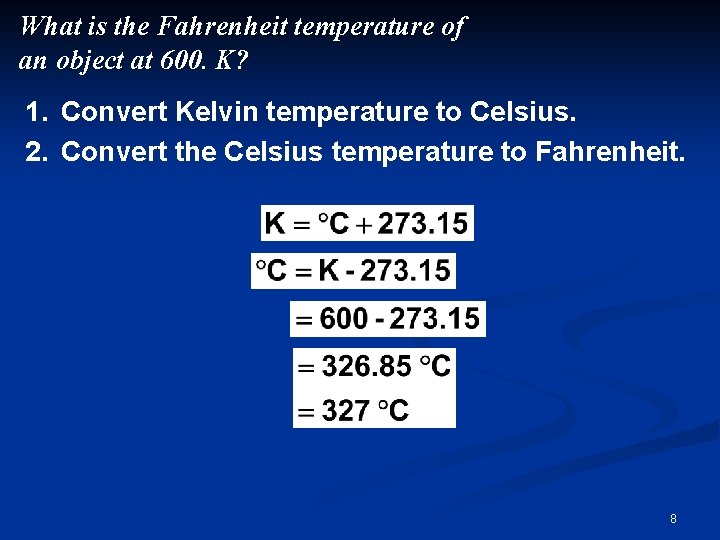
What is the Fahrenheit temperature of an object at 600. K? 1. Convert Kelvin temperature to Celsius. 2. Convert the Celsius temperature to Fahrenheit. 8

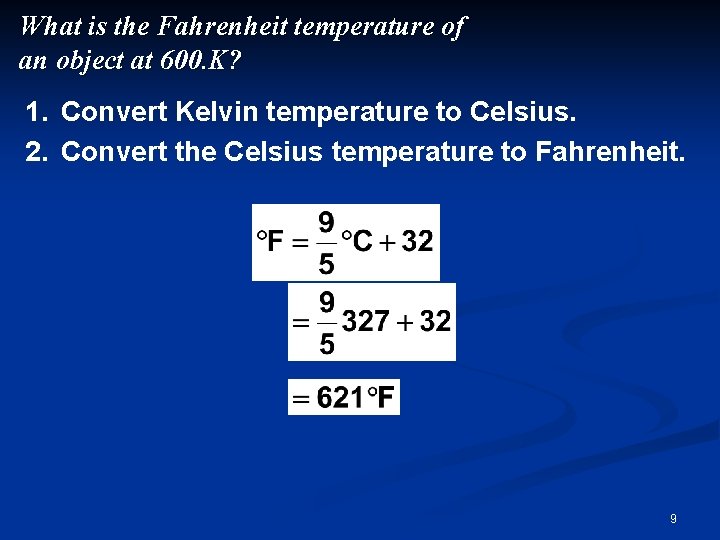
What is the Fahrenheit temperature of an object at 600. K? 1. Convert Kelvin temperature to Celsius. 2. Convert the Celsius temperature to Fahrenheit. 9

Thermal Energy n Thermal Energy the total energy of the particles in a material n KE - movement of particles n PE - forces within or between particles due to position n depends on temperature, mass, and type of substance n 10

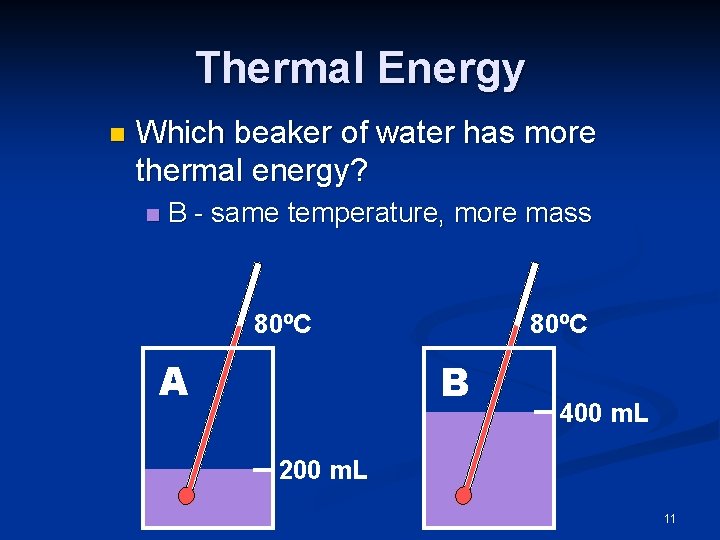
Thermal Energy n Which beaker of water has more thermal energy? n B - same temperature, more mass 80ºC A 80ºC B 400 m. L 200 m. L 11

Heat Transfer n Heat n n thermal energy that flows from a warmer material to a cooler material Like work, heat is. . . measured in joules (J) n a transfer of energy n 12

Three ways n Conduction-energy transfer through contact of particles n n Convection-energy transfer through mass movement of particles n n Water in contact with bottom of pot Warm water rising to top and cool water sinking to bottom of pot Radiation-energy transfer through waves n Light/heat from the sun 13

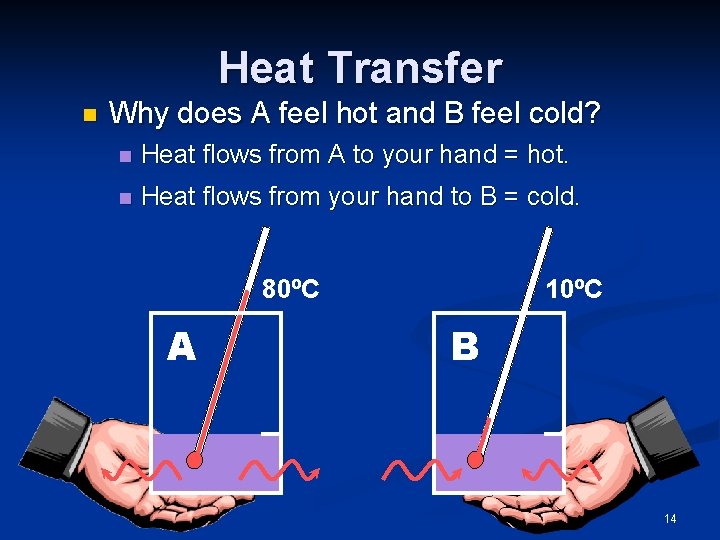
Heat Transfer n Why does A feel hot and B feel cold? n Heat flows from A to your hand = hot. n Heat flows from your hand to B = cold. 80ºC A 10ºC B 14

Heat Transfer n Specific Heat (Cp) amount of energy required to raise the temp. of 1 kg of material by 1 degree Kelvin n units: J/(kg·K) or J/(kg·°C) n 15

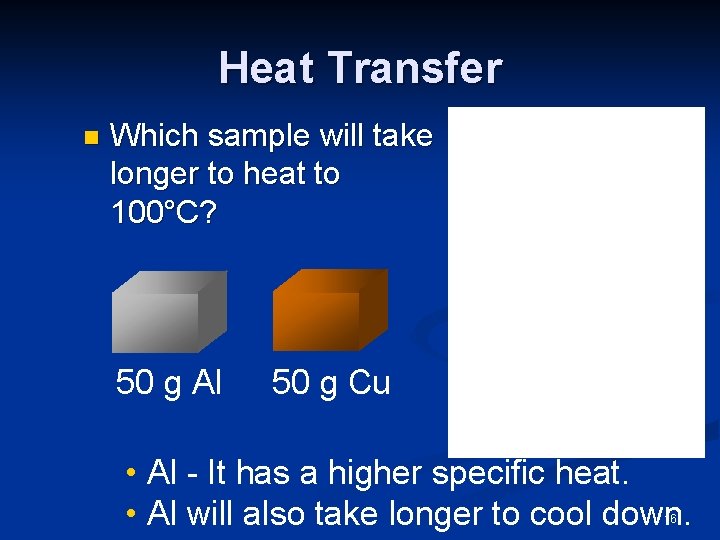
Heat Transfer n Which sample will take longer to heat to 100°C? 50 g Al 50 g Cu • Al - It has a higher specific heat. • Al will also take longer to cool down. 16

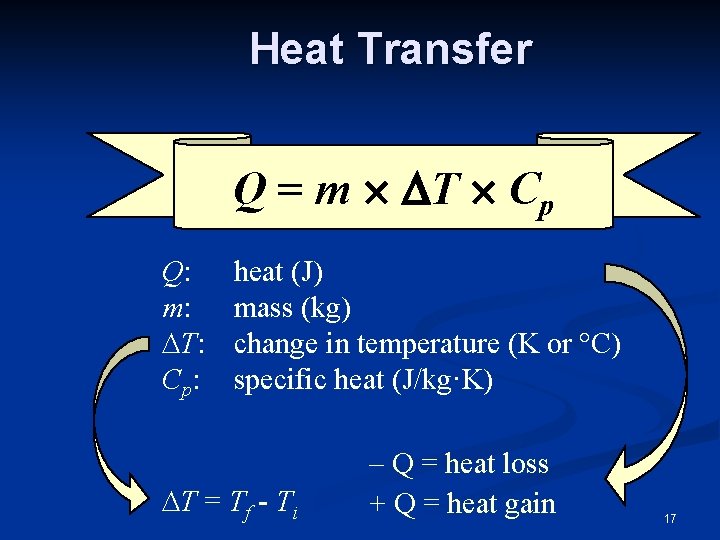
Heat Transfer Q = m T Cp Q: m: T: Cp: heat (J) mass (kg) change in temperature (K or °C) specific heat (J/kg·K) T = Tf - Ti – Q = heat loss + Q = heat gain 17

Heat Transfer n Calorimeter n device used to measure changes in thermal energy n in an insulated system, Coffee cup Calorimeter heat gained = heat lost 18

Heat Transfer n A 32 -g silver spoon cools from 60°C to 20°C. How much heat is lost by the spoon? GIVEN: m = 32 g Ti = 60°C Tf = 20°C Q=? Cp = 235 J/kg·K WORK: Q = m· T·Cp m = 32 g = 0. 032 kg T = 20°C - 60°C = – 40°C Q = (0. 032 kg)(40°C)(235 J/kg·K) Q = – 301 J 19

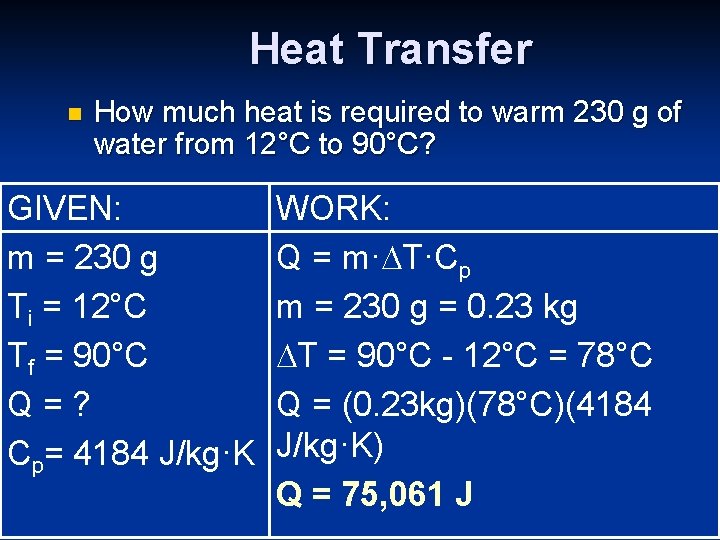
Heat Transfer n How much heat is required to warm 230 g of water from 12°C to 90°C? GIVEN: m = 230 g Ti = 12°C Tf = 90°C Q=? Cp= 4184 J/kg·K WORK: Q = m· T·Cp m = 230 g = 0. 23 kg T = 90°C - 12°C = 78°C Q = (0. 23 kg)(78°C)(4184 J/kg·K) Q = 75, 061 J 20