Energizer What are the 3 main particles within










- Slides: 23

Energizer What are the 3 main particles within the atom? List everything you know about each of these.

Unit 1 Part 3 n. The history of the atomic model n. The structure of the atom n. Reading the periodic table

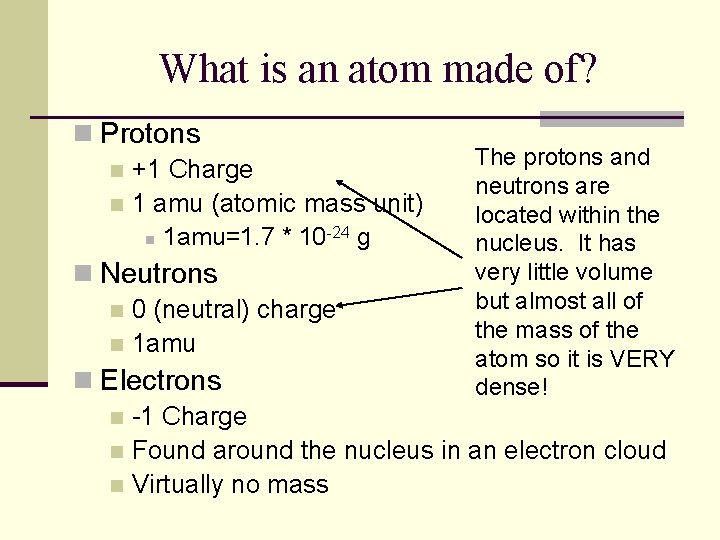
What is an atom made of? n Protons n +1 Charge n 1 amu (atomic mass unit) n 1 amu=1. 7 * 10 -24 g The protons and neutrons are located within the nucleus. It has very little volume but almost all of the mass of the atom so it is VERY dense! n Neutrons n 0 (neutral) charge n 1 amu n Electrons n -1 Charge n Found around the nucleus in an electron cloud n Virtually no mass

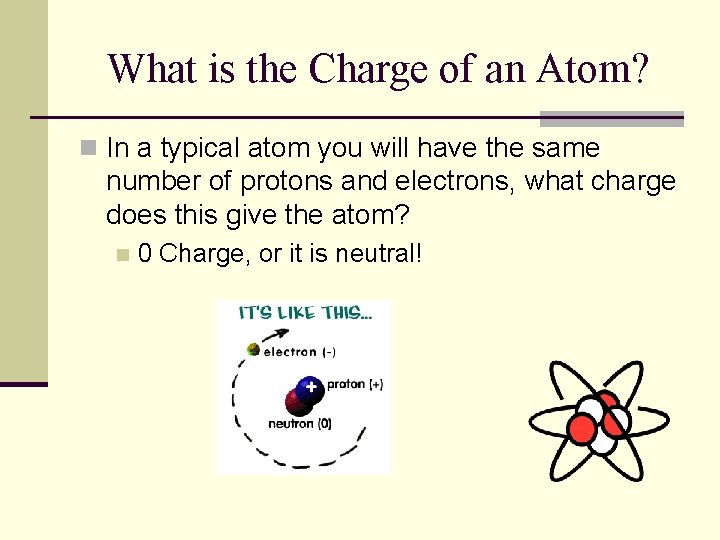
What is the Charge of an Atom? n In a typical atom you will have the same number of protons and electrons, what charge does this give the atom? n 0 Charge, or it is neutral!

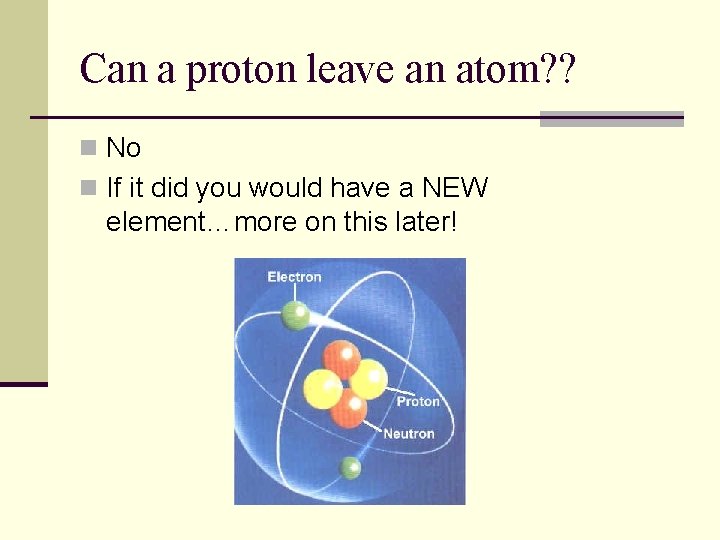
Can a proton leave an atom? ? n No n If it did you would have a NEW element…more on this later!

Can an electron leave or join an atom? ? n Yes! n This is called an Ion n If the atom is missing an electron it will be a Positive Ion. n If the atom gains and electron it will be a Negative Ion.

Can the number of neutrons change? n Yes! n This is called an isotope, it will cause the MASS of the atom to change

Reading the Periodic Table n Each element’s properties can be determined by the information in its “box” as well as its placement on the Table n See pages 768/69 to review the information!

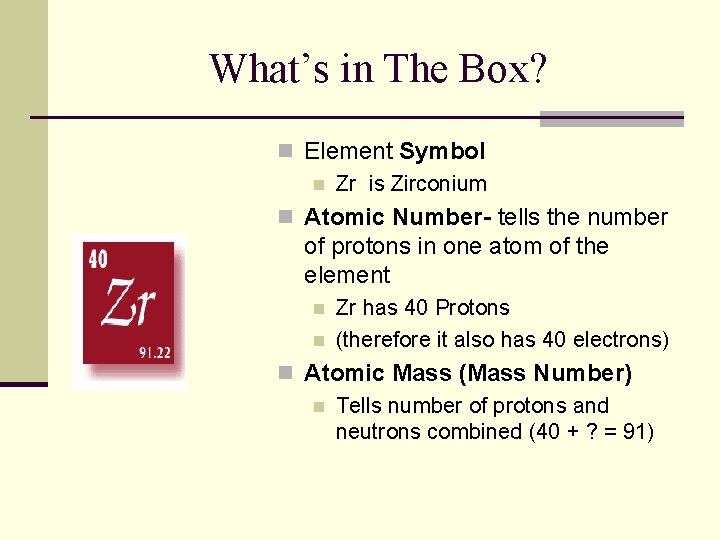
What’s in The Box? n Element Symbol n Zr is Zirconium n Atomic Number- tells the number of protons in one atom of the element n n Zr has 40 Protons (therefore it also has 40 electrons) n Atomic Mass (Mass Number) n Tells number of protons and neutrons combined (40 + ? = 91)

You Try… n My atomic number is 51, my name is… § Antimony n My atomic mass is 39. 95, my name is… § Argon n My atomic mass is 183. 85, my name is… § Tungsten n My atomic number is 102, my name is… § Nobelium

Some more… n My name is Sulfur and I have this many protons § 16 n My atomic number is 19, this is my name and how many electrons I have § Potassium, 19 electrons n My name is Cadmium, I have this many protons and neutrons combined § 112

Elements And Their Atoms n Each element has a unique atomic number n The number of protons for an element’s atom is constant and unique n The number of electrons will equal the number of protons UNLESS the atom is an ion n An element’s atom can have a different number of neutrons n Since the mass of an atom depends on the protons and neutrons, the mass of an element can change n To find neutrons, subtract the atomic # from the atomic mass

Isotope of Different Element n Element D has 6 protons and 7 neutrons Element F has 7 protons and 7 neutrons n Different Element n Element J has 27 protons and 32 neutrons Element L has 27 protons and 33 neutrons n Isotope n Element T has an atomic number of 20 and an atomic mass of 40. Element Z has an atomic number of 20 and an atomic mass of 41. n Isotope

For Example… n Look at the periodic table and find Carbon n What is the atomic number? n What is the mass number? n “Carbon 12” is the most common form of Carbon n But you’ve heard of Carbon 14…That is an isotope of Carbon – it has two more neutrons, adding 2 amu to its mass…but it still has only 6 protons n To name an Isotope n Element Name – Mass #

Forces In The Atom n Gravitational Force n Attractive force between all objects in the universe n Gravity depends upon mass of and distance between objects… n This force is not very strong in the atom n Electromagnetic Force n Opposites attract; likes repel n Protons & electrons n Electrons and Nucleus

More Forces in the Atom n Strong Force (the physicists got original naming this one) n Holds protons and neutrons together to make the nucleus n Greater than electromagnetic force between protons (since the protons would repel each other) n Weak Force – Yes, it’s a very weak force n Deals more with radioactive decay and nuclear fusion

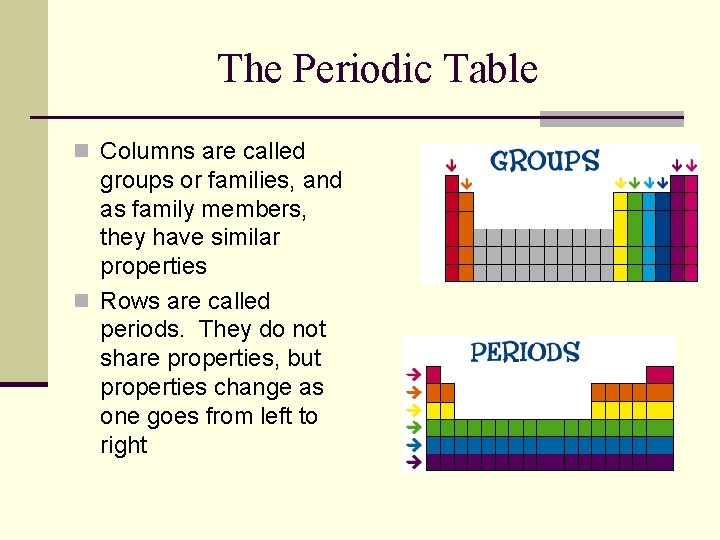
The Periodic Table n Columns are called groups or families, and as family members, they have similar properties n Rows are called periods. They do not share properties, but properties change as one goes from left to right

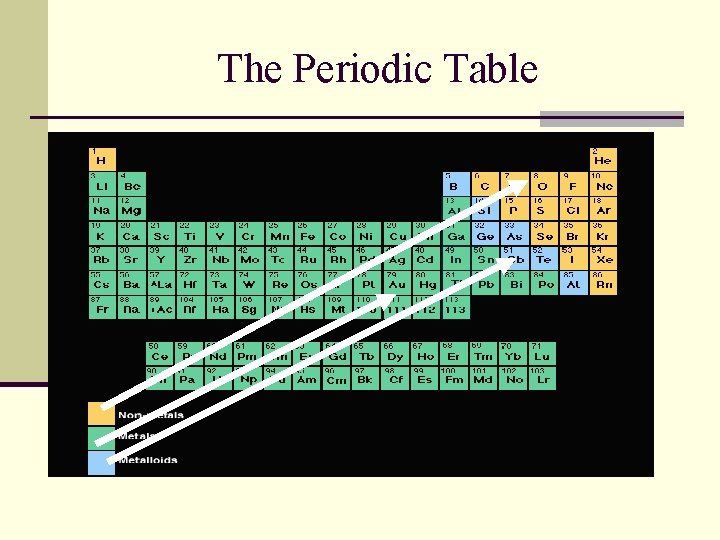
The Periodic Table

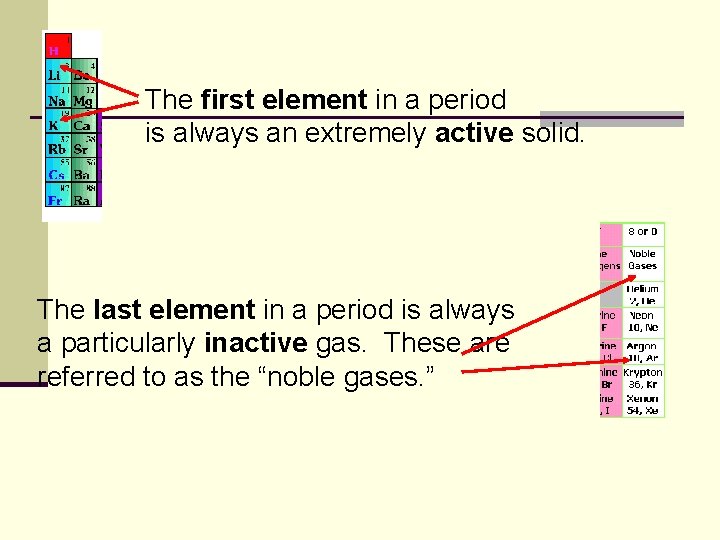
The first element in a period is always an extremely active solid. The last element in a period is always a particularly inactive gas. These are referred to as the “noble gases. ”

Warm Up n Using the periodic table, draw what you think a model of an atom of oxygen would look like n You should have 8 protons and 8 neutrons in the nucleus, and 8 electrons surrounding the nucleus.

Warm Up – Sept. 8 Find the Mystery Element n Find the atomic number of Hydrogen and add to it the atomic number of Beryllium; n Multiply Lithium’s atomic number by the answer in step 1; n Add five and divide by the atomic mass of Helium; n Add four to the answer in step 3; n The result is the atomic number of this element… n Fluorine

Find the Mystery Element n Multiply the protons of Lithium by the neutrons of Boron; n Add the electrons of Carbon; n Divide by the atomic number of Magnesium; n Add the protons of Arsenic; n Divide by the mass number of Lithium n The result is the atomic number of this element… n Boron, Atomic number

Another Mystery Element n Multiply the atomic number of Beryllium by the number of protons in Neon; n Subtract the atomic number of Carbon; n Add the number electrons of Helium; n Divide by the number of protons in Fluorine; n Subtract the number of electrons in Lithium; n The result is the atomic number of this element… n Hydrogen