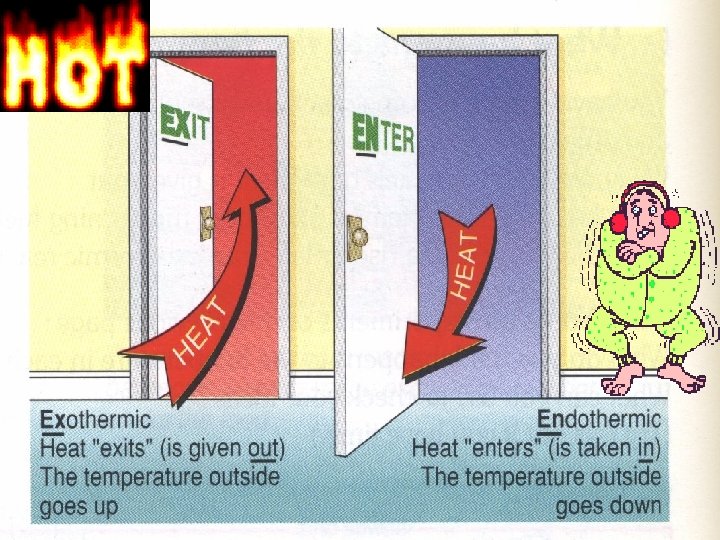
Energetics Exo endothermic reactions LOs What does endo










- Slides: 14

Energetics Exo & endothermic reactions LO’s: • What does endo & exo mean? • Application to reversible reactions


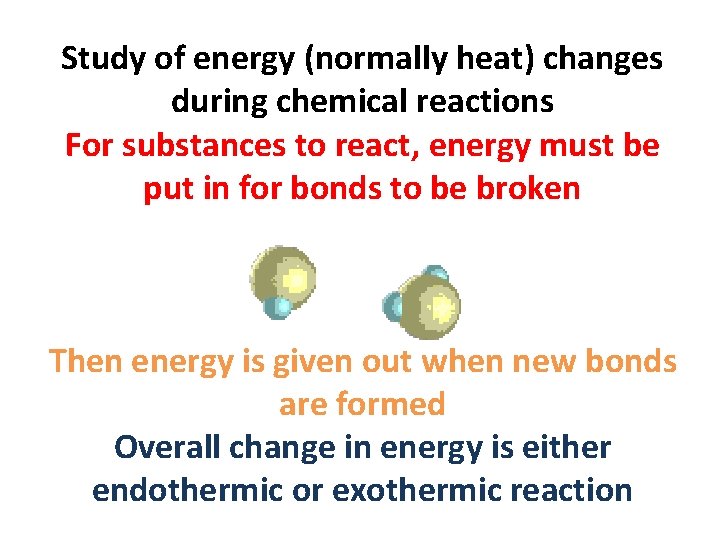
Study of energy (normally heat) changes during chemical reactions For substances to react, energy must be put in for bonds to be broken Then energy is given out when new bonds are formed Overall change in energy is either endothermic or exothermic reaction

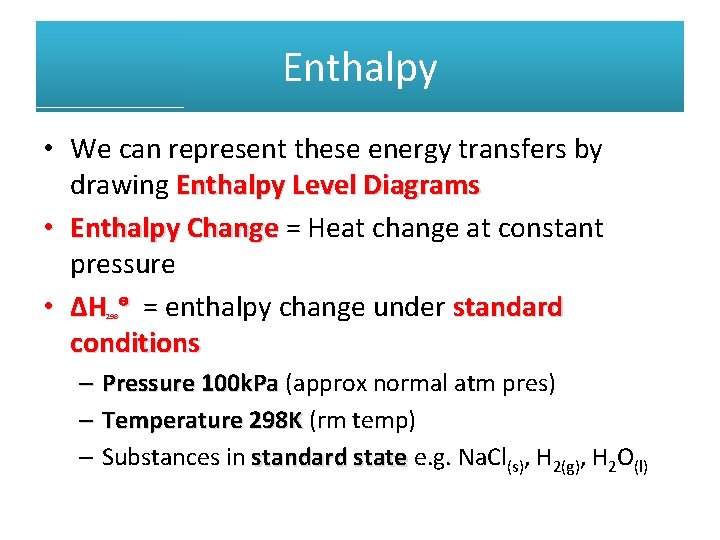
Enthalpy • We can represent these energy transfers by drawing Enthalpy Level Diagrams • Enthalpy Change = Heat change at constant pressure • ΔH ᶱ = enthalpy change under standard conditions 298 – Pressure 100 k. Pa (approx normal atm pres) – Temperature 298 K (rm temp) – Substances in standard state e. g. Na. Cl(s), H 2(g), H 2 O(l)

What type of reaction? • • • If the bonds in the REACTANTS have MORE energy than the PRODUCTS It is an EXOTHERMIC reaction Heat given OUT to surroundings Temperature INCREASES PRODUCT is more energetically stable

Exothermic Reactions Enthalpy REACTANTS The difference in energy is given out as heat, so the temperature surroundings goes up. Enthalpy change ΔH is -ve PRODUCTS Extent of reaction

What type of reaction? • • • If the bonds in the REACTANTS have LESS energy than the PRODUCTS It is an ENDOTHERMIC reaction Heat taken IN from surroundings Temperature FALLS PRODUCT is less energetically stable

Endothermic Reactions Enthalpy PRODUCTS REACTANTS Extent of reaction Extra energy needed to form the product is taken in from the surroundings, so the temperature of surroundings falls. Enthalpy change ΔH is +ve

Quantities • Enthalpy changes measured in k. Jmol - 1 • Always combined with a balanced equation • State symbols included e. g. CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(l) ΔHᶱ = -890 k. Jmol-1 • What does this tell me? • Means that when 1 mol CH 4 burnt in 2 mol O 2, 890 k. J are given out

What can this info tell you? Significance of this? Fuel Petrol Ethanol Methanol Hydrogen Enthalpy of combustion / k. Jmol-1 -5500 -1370 -730 -242 Mass of 1 mole / g Energy density / k. Jg-1 114 46 32 2 48. 2 29. 8 22. 8 121 • Write balanced symbol equation for combustion of hydrogen • What is the environmental significance of this? • What is the practical challenge of using hydrogen as a fuel?

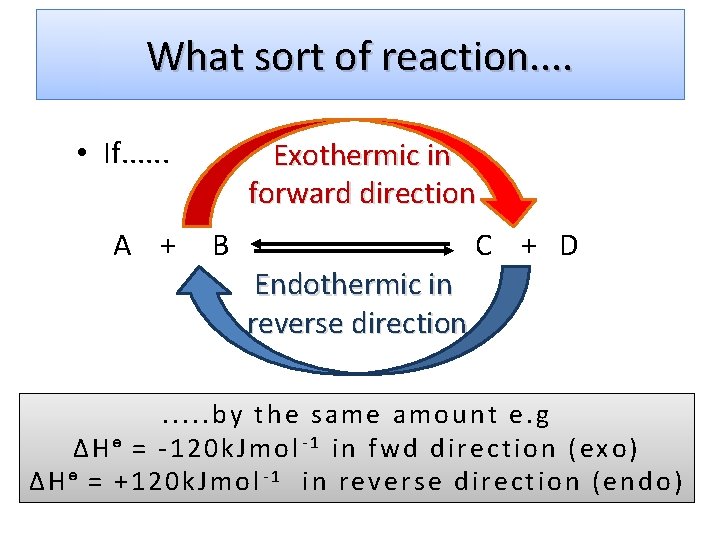
What sort of reaction. . • If. . . A + Exothermic in forward direction B C + D Endothermic in reverse direction . . . by the same amount e. g ΔHᶱ = -120 k. Jmol-1 in fwd direction (exo) ΔHᶱ = +120 k. Jmol-1 in reverse direction (endo)

Derive the definition Standard molar enthalpy of formation ΔHfᶱ • Enthalpy change when. . . Standard molar enthalpy of combustion ΔHcᶱ • Enthalpy change when. . . standard states constituent elements standard conditions formed compound one mole oxygen completely burned

Derive the definition Standard molar enthalpy of formation ΔHfᶱ • Enthalpy change when one mole of compound is formed from its constituent elements under standard conditions, all reactants & products in standard states Standard molar enthalpy of combustion ΔHcᶱ • Enthalpy change when one mole of compound is completely burned in oxygen under standard conditions, all reactants & products in standard states

Question time – handout front sheet • Complete the balanced equations according to the definitions of ΔHf & ΔHc